AloeninCAS# 38412-46-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38412-46-3 | SDF | Download SDF |
| PubChem ID | 162305 | Appearance | White powder |
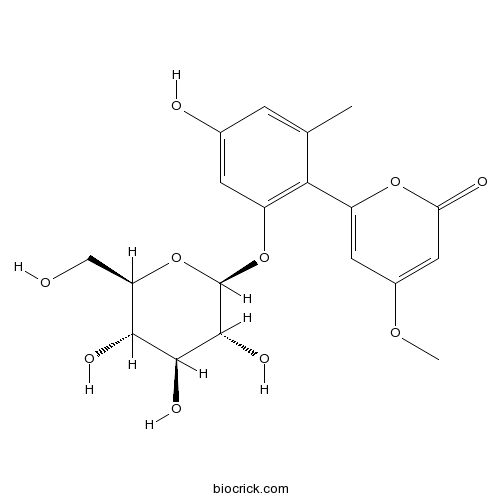
| Formula | C19H22O10 | M.Wt | 410.37 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Aloearbonaside; Aloenin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-[4-hydroxy-2-methyl-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-4-methoxypyran-2-one | ||
| SMILES | CC1=CC(=CC(=C1C2=CC(=CC(=O)O2)OC)OC3C(C(C(C(O3)CO)O)O)O)O | ||
| Standard InChIKey | KFJNVVJUICKJEQ-LQDZTQBFSA-N | ||
| Standard InChI | InChI=1S/C19H22O10/c1-8-3-9(21)4-11(15(8)12-5-10(26-2)6-14(22)27-12)28-19-18(25)17(24)16(23)13(7-20)29-19/h3-6,13,16-21,23-25H,7H2,1-2H3/t13-,16-,17+,18-,19-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Aloenin and aloe-emodin are active principles for inhibition of c-ADH and c-ALDH activities in vitro. |

Aloenin Dilution Calculator

Aloenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4368 mL | 12.1841 mL | 24.3683 mL | 48.7365 mL | 60.9206 mL |
| 5 mM | 0.4874 mL | 2.4368 mL | 4.8737 mL | 9.7473 mL | 12.1841 mL |
| 10 mM | 0.2437 mL | 1.2184 mL | 2.4368 mL | 4.8737 mL | 6.0921 mL |
| 50 mM | 0.0487 mL | 0.2437 mL | 0.4874 mL | 0.9747 mL | 1.2184 mL |
| 100 mM | 0.0244 mL | 0.1218 mL | 0.2437 mL | 0.4874 mL | 0.6092 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganaxolone
Catalog No.:BCC7397
CAS No.:38398-32-2
- Caudatin
Catalog No.:BCN5810
CAS No.:38395-02-7
- NSC 663284
Catalog No.:BCC7199
CAS No.:383907-43-5
- 3',4'-Anhydrovinblastine
Catalog No.:BCN2392
CAS No.:38390-45-3
- Taxinine
Catalog No.:BCN6944
CAS No.:3835-52-7
- Neuropeptide W-23 (human)
Catalog No.:BCC5961
CAS No.:383415-79-0
- UC 112
Catalog No.:BCC8042
CAS No.:383392-66-3
- Homovanillic Acid Sulfate
Catalog No.:BCN2226
CAS No.:38339-06-9
- 3-O-beta-D-Glucopyranosylplatycodigenin
Catalog No.:BCN7832
CAS No.:38337-25-6
- YE 120
Catalog No.:BCC6188
CAS No.:383124-82-1
- Minoxidil
Catalog No.:BCC4297
CAS No.:38304-91-5
- Naringenin trimethyl ether
Catalog No.:BCN5437
CAS No.:38302-15-7
- Altechromone A
Catalog No.:BCN7422
CAS No.:38412-47-4
- 3,4,4',7-Tetrahydroxyflavan
Catalog No.:BCN5438
CAS No.:38412-82-7
- Sclareol glycol
Catalog No.:BCN7007
CAS No.:38419-75-9
- H-Lys-OEt .2HCl
Catalog No.:BCC2980
CAS No.:3844-53-9
- Asatone
Catalog No.:BCN7761
CAS No.:38451-63-7
- Deacetyleupaserrin
Catalog No.:BCN7228
CAS No.:38456-39-2
- Eucannabinolide
Catalog No.:BCN7221
CAS No.:38458-58-1
- Crotafoline
Catalog No.:BCN2075
CAS No.:38494-87-0
- Tarafenacin
Catalog No.:BCC4147
CAS No.:385367-47-5
- Daphnicyclidin D
Catalog No.:BCN7081
CAS No.:385384-24-7
- Daphnicyclidin F
Catalog No.:BCN6400
CAS No.:385384-26-9
- Daphnicyclidin H
Catalog No.:BCN7080
CAS No.:385384-29-2
Novel type III polyketide synthases from Aloe arborescens.[Pubmed:19348024]
FEBS J. 2009 Apr;276(8):2391-401.
Aloe arborescens is a medicinal plant rich in aromatic polyketides, such as pharmaceutically important Aloenin (hexaketide), aloesin (heptaketide) and barbaloin (octaketide). Three novel type III polyketide synthases (PKS3, PKS4 and PKS5) were cloned and sequenced from the aloe plant by cDNA library screening. The enzymes share 85-96% amino acid sequence identity with the previously reported pentaketide chromone synthase and octaketide synthase. Recombinant PKS4 and PKS5 expressed in Escherichia coli were functionally identical to octaketide synthase, catalyzing the sequential condensations of eight molecules of malonyl-CoA to produce octaketides SEK4/SEK4b. As in the case of octaketide synthase, the enzymes are possibly involved in the biosynthesis of the octaketide barbaloin. On the other hand, PKS3 is a multifunctional enzyme that produces a heptaketide aloesone (i.e. the aglycone of aloesin) as a major product from seven molecules of malonyl-CoA. In addition, PKS3 also afforded a hexaketide pyrone (i.e. the precursor of Aloenin), a heptaketide 6-(2-acetyl-3,5-dihydroxybenzyl)-4-hydroxy-2-pyrone, a novel heptaketide 6-(2-(2,4-dihydroxy-6-methylphenyl)-2-oxoethyl)-4-hydroxy-2-pyrone and octaketides SEK4/SEK4b. This is the first demonstration of the enzymatic formation of the precursors of the pharmaceutically important aloesin and Aloenin by a wild-type PKS obtained from A. arborescens. Interestingly, the aloesone-forming activity was maximum at 50 degrees C, and the novel heptaketide pyrone was non-enzymatically converted to aloesone. In PKS3, the active-site residue 207, which is crucial for controlling the polyketide chain length depending on the steric bulk of the side chain, is uniquely substituted with Ala. Site-directed mutagenesis demonstrated that the A207G mutant dominantly produced the octaketides SEK4/SEK4b, whereas the A207M mutant yielded a pentaketide 5,7-dihydroxy-2-methylchromone.
Antiviral activity of Aloe hijazensis against some haemagglutinating viruses infection and its phytoconstituents.[Pubmed:22941477]
Arch Pharm Res. 2012 Aug;35(8):1347-54.
Evaluation of the antiviral activities of flowers, flower-peduncles, leaves, and roots of Aloe hijazensis against haemagglutinating viruses of avian paramyxovirus type-1 (APMV-1), avian influenza virus type A (AI-H5N1), Newcastle disease virus (NDV), and egg-drop syndrome virus (EDSV) in specific pathogen free (SPF) chicken embryos were carried out. Extract of the flowers and leaves showed relatively higher activity than the extracts of other plant parts. Thirteen compounds were isolated from both the flowers and flower-peduncles of A. hijazensis. The isolated compounds were classified into: five anthraquinones; ziganein, ziganein-5-methyl ether, aloesaponarin I, chrysophanol, aloe-emodin, one dihydroisocoumarin; feralolide, four flavonoids; homoplantaginin, isoorientin, luteolin 7-glucuronopyranoside, isovitexin, one phenolic acid; p-coumaric acid, the anthrone; barbaloin together with Aloenin. Eleven compounds were attributed to the flowers and seven to the flower-peduncles. Homoplantaginin and luteolin 7-glucuronopyranoside are reported here for the first time from Aloe spp. To the best of our knowledge, this is the first report on the chemical composition and biological activity of those plant parts.
New bioactive compounds from Aloe hijazensis.[Pubmed:19521919]
Nat Prod Res. 2009;23(11):1035-49.
The chemical constituents and biological activities of leaves and roots of Aloe hijazensis, collected in Saudi Arabia, are reported here for the first time. Twenty-two compounds were obtained, among them eight hydroxyquinones: aloe-emodin (1), emodin (2), chrysophanol (3), aloesaponarin II 3-methyl ether (4), ziganein (5), ziganein-5-methyl ether (6a), aloesaponarin I (7) and chrysophanein (8), the dihydro-isocoumarin feralolide (9), 4,7-dichloro-quinoline (10), the triterpene lupeol (11), the anthrone aloin (12), three Aloenin derivatives, Aloenin (13) ethylidene-Aloenin (14), and Aloenin B (15), four flavonoids, quercetin (16), kaempferol (17) cosmosiin (18) and isovitexin (19), and cinnamic acid (20) and two further analogues, caffeic acid (21) and ferulic acid (22). While 15 of the isolated compounds were found in the leaves, 12 were isolated from roots of the plant. Compounds 6a and 10 are reported as new natural constituents, while the compounds 4, 5, 8, and 18 are reported here for the first time from Aloe spp. The structures of the compounds were deduced by intensive studies of their UV, NMR, MS data and by comparison with related structures. The biological activity of plant extracts was studied against various microbial strains, and potent anti-bacterial and anti-fungal activities were found. [image omitted] [image omitted].
[A novel naphthalene derivative from Aloe barbadensis].[Pubmed:23888696]
Yao Xue Xue Bao. 2013 May;48(5):723-7.
To investigate the chemical constituents of A. barbadensis, aqueous extract of the plant was subjected to preparative medium pressure liquid chromatography (MPLC). The chemical structures were mainly determined by spectroscopic evidences (UV, IR, HR-MS, 1H NMR, 13C NMR, HSQC, 1H-1H COSY and HMBC) and chemical methods. A new O, O, O-triglucosylated naphthalene derivative, together with two known 6-phenyl-2-pyrone derivatives and four 5-methylchromones, were isolated and identified as 1-((3-((4- O-beta-D-glucopyranosyl)-beta-D-xylopyranosyloxymethyl)-1-hydroxy-8-alpha-L-rhamn opyranosyloxy)naphthalene-2-y])-ethanone (1), 10-O-beta-D-glucopyranosyl Aloenin (2), Aloenin B (3), aloesin (4), 8-C-glucosyl-(R)-aloesol (5), 8-C-glucosyl-7-O-methyl-(S)-aloesol (6), and isoaloeresin D (7). Compound 1 is a novel naphthalene derivative and named as aloveroside B, compounds 2-3 are isolated from this Aloe species for the first time.


