Iriflophenone 3-C-beta-D-glucopyranosideCAS# 104669-02-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104669-02-5 | SDF | Download SDF |
| PubChem ID | 184358 | Appearance | Light yellow powder |
| Formula | C19H20O10 | M.Wt | 408.4 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in methanol and water | ||
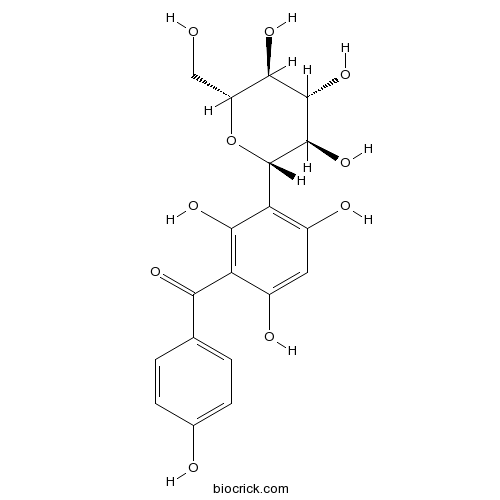
| Chemical Name | (4-hydroxyphenyl)-[2,4,6-trihydroxy-3-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]phenyl]methanone | ||
| SMILES | C1=CC(=CC=C1C(=O)C2=C(C(=C(C=C2O)O)C3C(C(C(C(O3)CO)O)O)O)O)O | ||
| Standard InChIKey | BZYKNVLTMWYEFA-ZJKJAXBQSA-N | ||
| Standard InChI | InChI=1S/C19H20O10/c20-6-11-15(25)17(27)18(28)19(29-11)13-10(23)5-9(22)12(16(13)26)14(24)7-1-3-8(21)4-2-7/h1-5,11,15,17-23,25-28H,6H2/t11-,15-,17+,18-,19+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Iriflophenone-3- C -glucoside has antioxidant activity, it presents no radical scavenging ability against DPPH , but scavenges ABTS + and peroxyl radicals (TEAC ABTS of 1.04 and TEAC ORAC of 3.61). 2. Iriflophenone-3-C-β-d-glucoside shows potent inhibitory activity against α-glucosidase. 3. Iriflophenone 3-C-β-D-glucoside and mangiferin which along with 3 β taraxerol and other sterols could be contributing to the cholesterol lowering activity. |

Iriflophenone 3-C-beta-D-glucopyranoside Dilution Calculator

Iriflophenone 3-C-beta-D-glucopyranoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4486 mL | 12.2429 mL | 24.4858 mL | 48.9716 mL | 61.2145 mL |
| 5 mM | 0.4897 mL | 2.4486 mL | 4.8972 mL | 9.7943 mL | 12.2429 mL |
| 10 mM | 0.2449 mL | 1.2243 mL | 2.4486 mL | 4.8972 mL | 6.1214 mL |
| 50 mM | 0.049 mL | 0.2449 mL | 0.4897 mL | 0.9794 mL | 1.2243 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2449 mL | 0.4897 mL | 0.6121 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7,3'-Di-O-methylorobol
Catalog No.:BCN6831
CAS No.:104668-88-4
- H-Ile-NH2.HCl
Catalog No.:BCC2962
CAS No.:10466-56-5
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Pramipexole
Catalog No.:BCC4467
CAS No.:104632-26-0
- Pramipexole dihydrochloride
Catalog No.:BCN2181
CAS No.:104632-25-9
- CGS 15943
Catalog No.:BCC7157
CAS No.:104615-18-1
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
- p-Menthan-3-one
Catalog No.:BCN3850
CAS No.:10458-14-7
- CAY10603
Catalog No.:BCC5542
CAS No.:1045792-66-2
- EC 23
Catalog No.:BCC6097
CAS No.:104561-41-3
- Bayogenin 3-O-beta-D-glucopyranoside
Catalog No.:BCN7868
CAS No.:104513-86-2
- Lupiwighteone
Catalog No.:BCN4045
CAS No.:104691-86-3
- Ganoderic acid K
Catalog No.:BCN3039
CAS No.:104700-95-0
- Ganoderol B
Catalog No.:BCN5859
CAS No.:104700-96-1
- Ganoderol A
Catalog No.:BCN5860
CAS No.:104700-97-2
- Ganoderal A
Catalog No.:BCN2451
CAS No.:104700-98-3
- Boc-D-Glu(OtBu)-OH
Catalog No.:BCC3395
CAS No.:104719-63-3
- 8-Phenyloctanol
Catalog No.:BCC8791
CAS No.:10472-97-6
- 6-O-α-Maltosyl-β-cyclodextrin
Catalog No.:BCC8075
CAS No.:104723-60-6
- Mayteine
Catalog No.:BCN3098
CAS No.:104736-05-2
- 4E-Deacetylchromolaenide 4'-O-acetate
Catalog No.:BCN7263
CAS No.:104736-09-6
- Ganoderic acid S
Catalog No.:BCN5861
CAS No.:104759-35-5
- Strontium chloride
Catalog No.:BCC7973
CAS No.:10476-85-4
Bio-assay guided isolation and identification of alpha-glucosidase inhibitors from the leaves of Aquilaria sinensis.[Pubmed:21215978]
Phytochemistry. 2011 Feb;72(2-3):242-7.
Eight alpha-glucosidase inhibitors including four new compounds were isolated from the 70% aqueous ethanolic extract of leaves of Aquilaria sinensis (Lour.) Gilg by activity-directed fractionation and purification processes. The ethanolic extract was first separated into petroleum ether, ethyl acetate, n-butanol and water soluble fractions and screened for inhibitory activity against alpha-glucosidase. Further activity-directed investigation lead to the isolation of four new compounds with moderate inhibitory activity, viz, aquilarisinin (1), aquilarisin (2), hypolaetin 5-O-beta-D-glucuronopyranoside (3) and aquilarixanthone (4) from the n-butanol fraction, and four known compounds showing potent activity including mangiferin (5), iriflophenone 2-O-alpha-L-rhamnopyranoside (6), iriflophenone 3-C-beta-D-glucoside (7) and iriflophenone 3,5-C-beta-D-diglucopyranoside (8) from the most potent ethyl acetate fraction. The structures of these compounds were determined by extensive spectroscopic analyses, including IR, UV, ESIMS, HRESIMS, 1D and 2D NMR.
Evaluation of Cholesterol-lowering Activity of Standardized Extract of Mangifera indica in Albino Wistar Rats.[Pubmed:28250649]
Pharmacognosy Res. 2017 Jan-Mar;9(1):21-26.
INTRODUCTION: Cholesterol lowering activity of Mangifera indica L. has been determined by earlier researchers and kernel, leaf and bark have shown significant activity. However, the specific cholesterol lowering activity of leaf methanol extract has not been determined. MATERIALS AND METHODS: The present study involved evaluation of cholesterol lowering potential of methanol extract of M. indica leaves using high cholesterol diet model in albino Wistar rats. The acute oral toxicity at a dose of 5000 mg/ kg body weight was also determined in female albino Wistar rats. Phytoconstituents Iriflophenone 3-C-beta-D-glucoside and mangiferin were quantified in methanol extracts of different varieties of mango leaves using high performance liquid chromatography. RESULTS AND DISCUSSION: Significant cholesterol lowering activity was observed with methanol extract of M. indica leaves, at dose of 90 mg/kg body weight in rats and it was also found to be safe at dose of 5000 mg/kg rat body. Iriflophenone 3-C-beta-D-glucoside and mangiferin were found to be in the range of 1.2 to 2.8% w/w and 3.9 to 4.6% w/w, respectively which along with 3 beta taraxerol and other sterols could be contributing to the cholesterol lowering activity of mango leaves extract. CONCLUSIONS: The phytosterols rich extract of Mangifera indica leaves is a good source of nutraceutical ingredient that have the potential to lower serum cholesterol levels. SUMMARY: The Mangifera indica leaves methanolic extract showed significant cholesterol lowering activity in high cholesterol diet induced hypercholesterolaemia model in rats when evaluated at a dose of 90 mg/kg rat body weight. The extract was found to contain Iriflophenone 3-C-beta-D-glucoside and mangiferin which along with 3 beta taraxerol and other sterols could be contributing to the cholesterol lowering activity.
Iriflophenone-3-C-glucoside from Cyclopia genistoides: isolation and quantitative comparison of antioxidant capacity with mangiferin and isomangiferin using on-line HPLC antioxidant assays.[Pubmed:24566268]
J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Mar 1;951-952:164-71.
The benzophenone, iriflophenone-3-C-glucoside, was isolated from Cyclopia genistoides using a combination of fluid-fluid extraction, high performance counter-current chromatography (HPCCC) and semi-preparative high performance liquid chromatography (HPLC). The microplate oxygen radical absorbance capacity (ORAC) assay, with fluorescein as probe, was adapted for use in an on-line HPLC configuration. The method was validated using a mixture of authentic standards including iriflophenone-3-C-glucoside, and the xanthones, mangiferin and isomangiferin. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was included in the mixture for calculation of Trolox equivalent antioxidant capacity (TEAC) values. Using the on-line HPLC-ORAC assay, as well as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS(+)) on-line assays, the antioxidant activity of iriflophenone-3-C-glucoside and isomangiferin was demonstrated for the first time. Iriflophenone-3-C-glucoside presented no radical scavenging ability against DPPH, but scavenged ABTS(+) and peroxyl radicals (TEACABTS of 1.04 and TEACORAC of 3.61). Isomangiferin showed slightly lower antioxidant capacity than mangiferin against DPPH (TEACDPPH of 0.57 vs. 0.62), but higher capacity against ABTS(+) (TEACABTS of 1.82 vs. 1.67) and peroxyl radical (TEACORAC of 4.14 vs. 3.69) than mangiferin. The on-line HPLC-ORAC assay was shown to be more sensitive for radical scavengers, but at the same time less selective for rapid radical scavengers than the DPPH assay.


