Tenovin-1SIRT2 inhibitor, activates p53 CAS# 380315-80-0 |
- SRT3190
Catalog No.:BCC1966
CAS No.:1204707-73-2
- PRIMA-1MET
Catalog No.:BCC2414
CAS No.:5291-32-7
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- Pifithrin-α (PFTα)
Catalog No.:BCC2241
CAS No.:63208-82-2
- NSC 319726
Catalog No.:BCC2242
CAS No.:71555-25-4
- JNJ-26854165 (Serdemetan)
Catalog No.:BCC2240
CAS No.:881202-45-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 380315-80-0 | SDF | Download SDF |
| PubChem ID | 1013376 | Appearance | Powder |
| Formula | C20H23N3O2S | M.Wt | 369.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (90.21 mM; Need ultrasonic) | ||
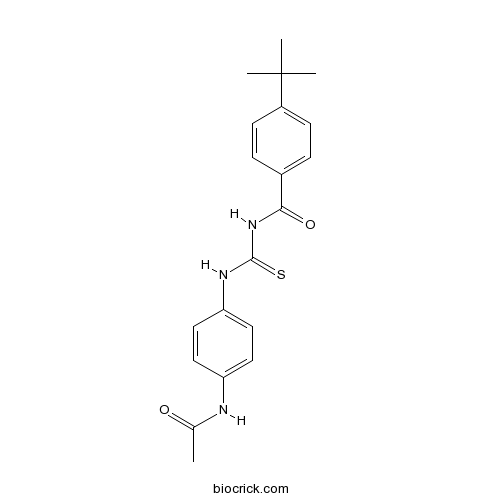
| Chemical Name | N-[(4-acetamidophenyl)carbamothioyl]-4-tert-butylbenzamide | ||
| SMILES | CC(=O)NC1=CC=C(C=C1)NC(=S)NC(=O)C2=CC=C(C=C2)C(C)(C)C | ||
| Standard InChIKey | WOWJIWFCOPZFGV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H23N3O2S/c1-13(24)21-16-9-11-17(12-10-16)22-19(26)23-18(25)14-5-7-15(8-6-14)20(2,3)4/h5-12H,1-4H3,(H,21,24)(H2,22,23,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | p53 activator that protects against MDM2-mediated p53 degradation. Elevates levels of p53 and p21CIP/WAF1 and induces expression from an endogenous p53-dependent promoter. Exhibits potent antiproliferative activity in vitro. |

Tenovin-1 Dilution Calculator

Tenovin-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7064 mL | 13.5318 mL | 27.0636 mL | 54.1272 mL | 67.659 mL |
| 5 mM | 0.5413 mL | 2.7064 mL | 5.4127 mL | 10.8254 mL | 13.5318 mL |
| 10 mM | 0.2706 mL | 1.3532 mL | 2.7064 mL | 5.4127 mL | 6.7659 mL |
| 50 mM | 0.0541 mL | 0.2706 mL | 0.5413 mL | 1.0825 mL | 1.3532 mL |
| 100 mM | 0.0271 mL | 0.1353 mL | 0.2706 mL | 0.5413 mL | 0.6766 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tenovin-1, a small molecule discovered by a cell-based screen, is a bio-active activator of p53 that elevates the levels of p53 protein, p53-downstream target p21CIP/WAF1 protein and mRNA and protects p53 from mdm2-mediated degradation with little impact on p53 synthesis. Tenovin-1 also exerts inhibition against human sirtuin 1 (SirT1) and sirtuin 2 (SirT2), two important members of the sirtuin family. Due to its insufficient solubility, an improved and water-soluble version of tenovin-1 has been found to decrease the peptide deacetylase activity of human SirT1 and SirT2 with values of 50% inhibition concentration IC50 of 21 μM and 10 μM respectively.
Reference
Lain S, Hollick JJ, Campbell J, Staples OD, Higgins M, Aoubala M, McCarthy A, Appleyard V, Murray KE, Baker L, Thompson A, Mathers J, Holland SJ, Stark MJ, Pass G, Woods J, Lane DP, Westwood NJ. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008 May;13(5):454-63. doi: 10.1016/j.ccr.2008.03.004.
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- LM 22A4
Catalog No.:BCC6239
CAS No.:37988-18-4
- Geranylacetone
Catalog No.:BCN7567
CAS No.:3796-70-1
- tenofovir diphosphate
Catalog No.:BCC6447
CAS No.:166403-66-3
- Tenofovir Alafenamide Fumarate
Catalog No.:BCC8067
CAS No.:379270-38-9
- Tenofovir alafenamide
Catalog No.:BCC8066
CAS No.:379270-37-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Cimifugin
Catalog No.:BCN5433
CAS No.:37921-38-3
- Jolkinolide B
Catalog No.:BCN2391
CAS No.:37905-08-1
- Jolkinolide A
Catalog No.:BCN3771
CAS No.:37905-07-0
- Scarlet 808
Catalog No.:BCC9139
CAS No.:3789-75-1
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
- NBMPR
Catalog No.:BCC7516
CAS No.:38048-32-7
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- 187-1, N-WASP inhibitor
Catalog No.:BCC5866
CAS No.:380488-27-7
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
- CMPDA
Catalog No.:BCC6151
CAS No.:380607-77-2
- [(pF)Phe4]Nociceptin(1-13)NH2
Catalog No.:BCC5778
CAS No.:380620-88-2
- Pterolactam
Catalog No.:BCN5435
CAS No.:38072-88-7
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- TACA
Catalog No.:BCC6564
CAS No.:38090-53-8
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
Induction of Nuclear Enlargement and Senescence by Sirtuin Inhibitors in Glioblastoma Cells.[Pubmed:27340387]
Immune Netw. 2016 Jun;16(3):183-8.
Sirtuin family members with lysine deacetylase activity are known to play an important role in anti-aging and longevity. Cellular senescence is one of the hallmarks of aging, and downregulation of sirtuin is reported to induce premature senescence. In this study, we investigated the effects of small-molecule sirtuin inhibitors on cellular senescence. Various small molecules such as Tenovin-1 and EX527 were employed for direct sirtuin activity inhibition. U251, SNB-75, and U87MG glioblastoma cells treated with sirtuin inhibitors exhibited phenotypes with nuclear enlargement. Furthermore, treatment of rat primary astrocytes with Tenovin-1 also increased the size of the nucleus. The activity of senescence-associated beta-galactosidase, a marker of cellular senescence, was induced by Tenovin-1 and EX527 treatment in U87MG glioblastoma cells. Consistent with the senescent phenotype, treatment with Tenovin-1 increased p53 expression in U87MG cells. This study demonstrated the senescence-inducing effect of sirtuin inhibitors, which are potentially useful tools for senescence research.
The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells.[Pubmed:26350565]
Mol Cell Biochem. 2015 Dec;410(1-2):187-95.
Estrogen is synthesized from cholesterol and high cholesterol levels are suggested to be associated with increased risk of estrogen receptor(ER)-positive breast cancer. The cholesterol metabolite 27-hydroxycholesterol (27-OHC) was recently identified as a selective estrogen receptor modulator (SERM) and may therefore impact breast cancer progression. However, the mechanisms by which 27-OHC may contribute to breast cancer are not all known. We determined the extent to which 27-OHC regulates cell proliferation in MCF7 ER-positive breast cancer cell line involving the tumor suppressor protein p53. We found that treatment of MCF7 cells with 27-OHC resulted reduced p53 transcriptional activity. Conversely, treatment of the ER-negative MDA-MB 231 cells with 27-OHC induced no significant change in p53 activity. Exposure of MCF7 cells to 27-OHC was also associated with increased protein levels of the E3 ubiquitin protein ligase MDM2 and decreased levels of p53. Moreover, 27-OHC also enhanced physical interaction between p53 and MDM2. Furthermore, 27-OHC-induced proliferation was attenuated using either the p53 activator Tenovin-1 or the MDM2 inhibitor Nutlin-3 and Mdm2 siRNA. Taken together, our results indicate that 27-OHC may contribute to ER-positive breast cancer progression by disrupting constitutive p53 signaling in an MDM2-dependent manner.
Expression of sirtuin 1 and 2 is associated with poor prognosis in non-small cell lung cancer patients.[Pubmed:25915617]
PLoS One. 2015 Apr 27;10(4):e0124670.
BACKGROUND: Sirtuin 1 (SIRT1) and sirtuin 2 (SIRT2) are NAD+-dependent protein deacetylases involved in the regulation of key cancer-associated genes. In this study we evaluated the relevance of these deacetylases in lung cancer biology. MATERIAL AND METHODS: Protein levels of SIRT1 and SIRT2 were determined in non-small cell lung cancer (NSCLC) cell lines and primary tumors from 105 patients. Changes in proliferation were assessed after SIRT1 and SIRT2 downregulation in lung cancer cell lines using siRNA-mediated technology or Tenovin-1, a SIRT1 and SIRT2 inhibitor. RESULTS: High SIRT1 and SIRT2 protein levels were found in NSCLC cell lines compared with non-tumor lung epithelial cells. The expression of SIRT1 and SIRT2 proteins was also significantly higher in lung primary tumors than in normal tissue (P<0.001 for both sirtuins). Stronger nuclear SIRT1 staining was observed in adenocarcinomas than in squamous cell carcinomas (P=0.033). Interestingly, in NSCLC patients, high SIRT1 and SIRT2 expression levels were associated with shorter recurrence-free survival (P=0.04 and P=0.007, respectively). Moreover, the combination of high SIRT1 and SIRT2 expression was an independent prognostic factor for shorter recurrence-free survival (P=0.002) and overall survival (P=0.022). In vitro studies showed that SIRT1 and/or SIRT2 downregulation significantly decreased proliferation of NSCLC. CONCLUSIONS: Our results support the hypothesis that SIRT1 and SIRT2 have a protumorigenic role in lung cancer, promoting cell proliferation. Moreover, the expression of these proteins is associated with poor prognosis in NSCLC patients and may help to identify those NSCLC patients with high risk of recurrence that could benefit from adjuvant therapy after resection.
Sirtuin deacetylases: a new target for melanoma management.[Pubmed:25486469]
Cell Cycle. 2014;13(18):2821-6.
Melanoma continues to cause more deaths than any other skin cancer, necessitating the development of new avenues of treatment. One promising new opportunity comes in the form of mechanism-based therapeutic targets. We recently reported the overexpression and delocalization of the class III histone deacetylase SIRT1 in melanoma, and demonstrated that its small molecule inhibition via Tenovin-1 decreased cell growth and viability of melanoma cells, possibly by a p53 mediated induction of p21. Here, we support our data using additional SIRT inhibitors, viz. Sirtinol and Ex-527, which suggests possible benefits of concomitantly inhibiting more than one Sirtuin for an effective cancer management strategy. This "Extra View" paper also includes a discussion of our results in the context of similar recent and concurrent studies. Furthermore, we expand upon our findings in an analysis of new research that may link the cellular localization and growth effects of SIRT1 with the PI3K signaling pathway.
Combining p53 stabilizers with metformin induces synergistic apoptosis through regulation of energy metabolism in castration-resistant prostate cancer.[Pubmed:26900800]
Cell Cycle. 2016;15(6):840-9.
Since altered energy metabolism is a hallmark of cancer, many drugs targeting metabolic pathways are in active clinical trials. The tumor suppressor p53 is often inactivated in cancer, either through downregulation of protein or loss-of-function mutations. As such, stabilization of p53 is considered as one promising approach to treat those cancers carrying wild type (WT) p53. Herein, SIRT1 inhibitor Tenovin-1 and polo-like kinase 1 (Plk1) inhibitor BI2536 were used to stabilize p53. We found that both Tennovin-1 and BI2536 increased the anti-neoplastic activity of metformin, an inhibitor of oxidative phosphorylation, in a p53 dependent manner. Since p53 has also been shown to regulate metabolic pathways, we further analyzed glycolysis and oxidative phosphorylation upon drug treatments. We showed that both Tennovin-1 and BI2536 rescued metformin-induced glycolysis and that both Tennovin-1 and BI2536 potentiated metformin-associated inhibition of oxidative phosphorylation. Of significance, castration-resistant prostate cancer (CRPC) C4-2 cells show a much more robust response to the combination treatment than the parental androgen-dependent prostate cancer LNCaP cells, indicating that targeting energy metabolism with metformin plus p53 stabilizers might be a valid approach to treat CRPC carrying WT p53.
Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator.[Pubmed:18455128]
Cancer Cell. 2008 May;13(5):454-63.
We have carried out a cell-based screen aimed at discovering small molecules that activate p53 and have the potential to decrease tumor growth. Here, we describe one of our hit compounds, Tenovin-1, along with a more water-soluble analog, tenovin-6. Via a yeast genetic screen, biochemical assays, and target validation studies in mammalian cells, we show that tenovins act through inhibition of the protein-deacetylating activities of SirT1 and SirT2, two important members of the sirtuin family. Tenovins are active on mammalian cells at one-digit micromolar concentrations and decrease tumor growth in vivo as single agents. This underscores the utility of these compounds as biological tools for the study of sirtuin function as well as their potential therapeutic interest.


