Jolkinolide ACAS# 37905-07-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 37905-07-0 | SDF | Download SDF |
| PubChem ID | 161953 | Appearance | Cryst. |
| Formula | C20H26O3 | M.Wt | 314.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
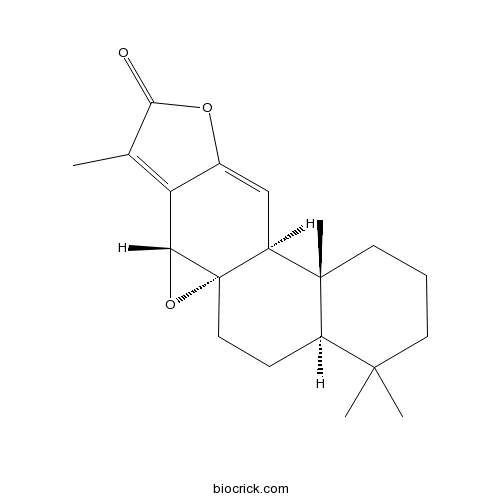
| SMILES | CC1=C2C3C4(O3)CCC5C(CCCC5(C4C=C2OC1=O)C)(C)C | ||
| Standard InChIKey | OYXDHOVYZKWSRM-PHJMNMFVSA-N | ||
| Standard InChI | InChI=1S/C20H26O3/c1-11-15-12(22-17(11)21)10-14-19(4)8-5-7-18(2,3)13(19)6-9-20(14)16(15)23-20/h10,13-14,16H,5-9H2,1-4H3/t13-,14+,16-,19-,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Jolkinolide A has anti-tumor activity. It inhibited VEGF expression in A549 cells through the inhibition of the Akt-STAT3-mTOR signaling pathway, and directly inhibited the proliferation and migration of HUVECs. |
| Targets | VEGF | Akt | Akt | STAT | mTOR |
| In vitro | Jolkinolide A and Jolkinolide B Inhibit Proliferation of A549 Cells and Activity of Human Umbilical Vein Endothelial Cells.[Pubmed: 28087861]Medical Science Monitor, 2017, 23:223-237.Jolkinolide A (JA) and Jolkinolide B (JB) are diterpenoids extracted from the roots of Euphorbia fischeriana Steud and have been shown to have anti-tumor activity. However, their effects on the ability of tumor cells to invade blood vessels and metastasize remain largely unknown. Investigations into the effects of JA and JB on the angiogenesis of tumor tissues may facilitate the identification of new natural drugs with anti-tumor growth and metastasis activities. Cytotoxic diterpenoids from the roots of Euphorbia ebracteolata.[Pubmed: 15856412]Planta Medica, 2005, 71(4):349-354.
|
| Structure Identification | Chinese Journal of Pharmaceutical Analysis (2006) 26(9) 1204-1206.HPLC determination of jolkinolide A and jolkinolide B in Chinese herb medicine" Lang- du"[Reference: WebLink]To establish a method for the determination of two diterpenoide lactones, Jolkinolide A and jolkinolide B in Chinese herbal medicine "Lang -du" by HPLC. |

Jolkinolide A Dilution Calculator

Jolkinolide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1807 mL | 15.9033 mL | 31.8066 mL | 63.6132 mL | 79.5165 mL |
| 5 mM | 0.6361 mL | 3.1807 mL | 6.3613 mL | 12.7226 mL | 15.9033 mL |
| 10 mM | 0.3181 mL | 1.5903 mL | 3.1807 mL | 6.3613 mL | 7.9517 mL |
| 50 mM | 0.0636 mL | 0.3181 mL | 0.6361 mL | 1.2723 mL | 1.5903 mL |
| 100 mM | 0.0318 mL | 0.159 mL | 0.3181 mL | 0.6361 mL | 0.7952 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Scarlet 808
Catalog No.:BCC9139
CAS No.:3789-75-1
- Ro 08-2750
Catalog No.:BCC7307
CAS No.:37854-59-4
- Nepodin
Catalog No.:BCN6894
CAS No.:3785-24-8
- Germacrene D
Catalog No.:BCN3851
CAS No.:37839-63-7
- Phaseollidin
Catalog No.:BCN5432
CAS No.:37831-70-2
- ACDPP hydrochloride
Catalog No.:BCC7302
CAS No.:37804-11-8
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- Cimigenol
Catalog No.:BCC8149
CAS No.:3779-59-7
- Boc-D-Pro-OH
Catalog No.:BCC3437
CAS No.:37784-17-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
- 7''-O-Methylsciadopitysin
Catalog No.:BCN4018
CAS No.:3778-25-4
- Preladenant
Catalog No.:BCC1868
CAS No.:377727-87-2
- Jolkinolide B
Catalog No.:BCN2391
CAS No.:37905-08-1
- Cimifugin
Catalog No.:BCN5433
CAS No.:37921-38-3
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Tenofovir alafenamide
Catalog No.:BCC8066
CAS No.:379270-37-8
- Tenofovir Alafenamide Fumarate
Catalog No.:BCC8067
CAS No.:379270-38-9
- tenofovir diphosphate
Catalog No.:BCC6447
CAS No.:166403-66-3
- Geranylacetone
Catalog No.:BCN7567
CAS No.:3796-70-1
- LM 22A4
Catalog No.:BCC6239
CAS No.:37988-18-4
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
Jolkinolide A and Jolkinolide B Inhibit Proliferation of A549 Cells and Activity of Human Umbilical Vein Endothelial Cells.[Pubmed:28087861]
Med Sci Monit. 2017 Jan 14;23:223-237.
BACKGROUND Jolkinolide A (JA) and Jolkinolide B (JB) are diterpenoids extracted from the roots of Euphorbia fischeriana Steud and have been shown to have anti-tumor activity. However, their effects on the ability of tumor cells to invade blood vessels and metastasize remain largely unknown. Investigations into the effects of JA and JB on the angiogenesis of tumor tissues may facilitate the identification of new natural drugs with anti-tumor growth and metastasis activities. MATERIAL AND METHODS We used different concentrations of JA and JB (20 mug/ml, 40 mug/ml, 60 mug/ml, 80 mug/ml, and 100 mug/ml) to stimulate A549 cells and then studied the effects on the growth and metastasis of lung cancers. In addition, we used conditional media from A549 cells (A549-CM) stimulated by either JA or JB in different concentrations to culture human umbilical vein endothelial cells (HUVECs). RESULTS We found that both JA and JB significantly inhibited the Akt-STAT3-mTOR signaling pathway and reduced the expression of VEGF in A549 cells, but JB exhibited more significant inhibitory effects than JA. The JB-stimulated A549 cell conditional media had a greater inhibitory effect on the proliferation and migration of HUVECs than did the conditional media of JA-stimulated A549 cells. This effect gradually increased with increasing concentrations of either type of Jolkinolide. CONCLUSIONS Our results suggest that JA and JB inhibited VEGF expression in A549 cells through the inhibition of the Akt-STAT3-mTOR signaling pathway, and directly inhibited the proliferation and migration of HUVECs. These findings are of great significance for the development of new plant-derived chemotherapy agents for the treatment of cancer.
ent-Abietane Lactones from Euphorbia.[Pubmed:27670580]
Mini Rev Med Chem. 2017;17(4):380-397.
Extracted from Euphorbia, ent-Abietane lactones can be classified into several categories, such as Jolkinolides and Helioscopinolides, according to their structural features. The study of ent- Abietane lactones could date back to 1972, when Jolkinolide A and B were first isolated from Euphorbia jolkini Boiss. Since then, many other ent-Abietane lactones have been extracted from different species of Euphorbia. Their bio-activities include anti-tumor activity, anti-inflammatory activity as well as anti-bacterial activity. Among them, derivatives of Jolkinolide B draw the most attention for their high anti-tumor activity. There are many studies focus on the syntheses of Jolkinolides. In 1989, the first and efficient synthesis of Jolkinolides was accomplished by Katsumura et al. Their strategy to construct the last ring of Jolkinolides contributes a lot to the following studies. In the following thirty years, there are also other semi-syntheses of Jolkinolides conducted, basing on different starting materials. In this review, we will give a brief clarification of ent-Abietane lactones, as well as their bio-activities and syntheses.


