IfosfamideCytostatic agent CAS# 3778-73-2 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Carboplatin
Catalog No.:BCC1170
CAS No.:41575-94-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3778-73-2 | SDF | Download SDF |
| PubChem ID | 3690 | Appearance | Powder |
| Formula | C7H15Cl2N2O2P | M.Wt | 261.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (191.50 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N,3-bis(2-chloroethyl)-2-oxo-1,3,2$l^{5}-oxazaphosphinan-2-amine | ||
| SMILES | C1CN(P(=O)(OC1)NCCCl)CCCl | ||
| Standard InChIKey | HOMGKSMUEGBAAB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H15Cl2N2O2P/c8-2-4-10-14(12)11(6-3-9)5-1-7-13-14/h1-7H2,(H,10,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ifosfamide Dilution Calculator

Ifosfamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8301 mL | 19.1505 mL | 38.301 mL | 76.6019 mL | 95.7524 mL |
| 5 mM | 0.766 mL | 3.8301 mL | 7.6602 mL | 15.3204 mL | 19.1505 mL |
| 10 mM | 0.383 mL | 1.915 mL | 3.8301 mL | 7.6602 mL | 9.5752 mL |
| 50 mM | 0.0766 mL | 0.383 mL | 0.766 mL | 1.532 mL | 1.915 mL |
| 100 mM | 0.0383 mL | 0.1915 mL | 0.383 mL | 0.766 mL | 0.9575 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
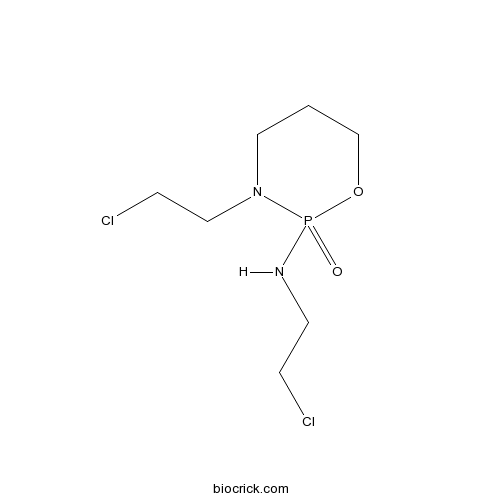
Ifosfamide is a cytostatic agent that is structurally related to cyclophosphamide.
- 7''-O-Methylsciadopitysin
Catalog No.:BCN4018
CAS No.:3778-25-4
- Preladenant
Catalog No.:BCC1868
CAS No.:377727-87-2
- 2-Pentylfuran
Catalog No.:BCN3799
CAS No.:3777-69-3
- Zaprinast
Catalog No.:BCC6859
CAS No.:37762-06-4
- Enmein
Catalog No.:BCN3392
CAS No.:3776-39-4
- Iristectorin A
Catalog No.:BCN8221
CAS No.:37744-61-9
- Questin
Catalog No.:BCN7446
CAS No.:3774-64-9
- 2-Chloro-N6-cyclopentyladenosine
Catalog No.:BCC7161
CAS No.:37739-05-2
- Boc-Cha-OH
Catalog No.:BCC2661
CAS No.:37736-82-6
- Rhodojaponin V
Catalog No.:BCN2807
CAS No.:37720-86-8
- Totaradiol
Catalog No.:BCN5431
CAS No.:3772-56-3
- Dehydroabietinol
Catalog No.:BCN5430
CAS No.:3772-55-2
- Boc-D-Pro-OH
Catalog No.:BCC3437
CAS No.:37784-17-1
- Cimigenol
Catalog No.:BCC8149
CAS No.:3779-59-7
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- ACDPP hydrochloride
Catalog No.:BCC7302
CAS No.:37804-11-8
- Phaseollidin
Catalog No.:BCN5432
CAS No.:37831-70-2
- Germacrene D
Catalog No.:BCN3851
CAS No.:37839-63-7
- Nepodin
Catalog No.:BCN6894
CAS No.:3785-24-8
- Ro 08-2750
Catalog No.:BCC7307
CAS No.:37854-59-4
- Scarlet 808
Catalog No.:BCC9139
CAS No.:3789-75-1
- Jolkinolide A
Catalog No.:BCN3771
CAS No.:37905-07-0
- Jolkinolide B
Catalog No.:BCN2391
CAS No.:37905-08-1
- Cimifugin
Catalog No.:BCN5433
CAS No.:37921-38-3
Synthesis of novel monomeric graphene quantum dots and corresponding nanocomposite with molecularly imprinted polymer for electrochemical detection of an anticancerous ifosfamide drug.[Pubmed:28237900]
Biosens Bioelectron. 2017 Aug 15;94:1-9.
This paper reports a typical synthesis of a nanocomposite of functionalized graphene quantum dots and imprinted polymer at the surface of screen-printed carbon electrode using N-acryloyl-4-aminobenzamide, as a functional monomer, and an anticancerous drug, Ifosfamide, as a print molecule (test analyte). Herein, graphene quantum dots in nanocomposite practically induced the electrocatalytic activity by lowering the oxidation overpotential of test analyte and thereby amplifying electronic transmission, without any interfacial barrier in between the film and the electrode surface. The differential pulse anodic stripping signal at functionalized graphene quantum dots based imprinted sensor was realized to be about 3- and 7-fold higher as compared to the traditionally made imprinted polymers prepared in the presence and the absence of graphene quantum dots (un-functionalized), respectively. This may be attributed to a pertinent synergism in between the positively charged functionalized graphene quantum dots in the film and the target analyte toward the enhancement of electro-conductivity of the film and thereby the electrode kinetics. In fact, the covalent attachment of graphene quantum dots with N-acryloyl-4-aminobenzamide molecules might exert an extended conjugation at their interface facilitating electro conducting to render the channelized pathways for the electron transport. The proposed sensor is practically applicable to the ultratrace evaluation of Ifosfamide in real (biological/pharmaceutical) samples with detection limit as low as 0.11ngmL(-1) (S/N=3), without any matrix effect, cross-reactivity, and false-positives.
VIP (etoposide, ifosfamide, and cisplatin) in patients with previously treated soft tissue sarcoma.[Pubmed:28121937]
Medicine (Baltimore). 2017 Jan;96(4):e5942.
We retrospectively reviewed outcomes of treatment with VIP (combination of etoposide, Ifosfamide, and cisplatin) in patients with previously treated soft tissue sarcoma (STS).We analyzed the medical records of patients with advanced or relapsed STS who had undergone VIP treatment as second-line or more chemotherapy between January 2000 and December 2015. The patients were treated with a combination of etoposide (100 mg/m for 5 days), Ifosfamide (2000 mg/m for 2 days), and cisplatin (20 mg/m for 5 days) once every 4 weeks. Treatment response, progression-free survival (PFS), and overall survival (OS) were analyzed in all patients and between responder and nonresponder groups (responders showed a tumor response to any prior systemic chemotherapy before VIP).Twenty-four patients with a median age of 50 years (range: 20-68 years) were treated with VIP. Eleven (45.8%) patients were male and 7 (29.2%) received 2 or more chemotherapy regimens before VIP. Median PFS was 3.7 months (95% confidence interval [CI], 1.3-6.1 months) and median OS was 10.0 months (95% CI, 6.6-13.5). The overall response rate was 37.5%, and the disease control rate was 50%. The responder group showed better PFS (7.7 months vs 3.0 months; P = 0.101) and significantly improved OS (11.0 months vs 8.8 months; P = 0.039) compared to those of nonresponders. All patients reported some grade of hematological toxicity. The most frequently encountered hematological toxicity was neutropenia (any grade, 77.7%; grade 3 or 4, 74.0%).VIP might be effective in patients with previously treated STS.
Induction chemotherapy with cisplatin and ifosfamide in locally advanced inoperable squamous cell carcinoma of the head and neck: A single-institution experience.[Pubmed:28244461]
Indian J Cancer. 2016 Jul-Sep;53(3):372-376.
BACKGROUND: Induction chemotherapy (ICT) in patients with head and neck cancer has been studied since a long time. The addition of taxanes to the cisplatin and 5-fluorouracil (5FU) (PF) regimen results in superior antitumor activity. We did this study to see the response and toxicity of ICT with cisplatin and Ifosfamide followed by concurrent chemoradiotherapy (CRT) in locally advanced, unresectable squamous cell carcinoma of head and neck (SCCHN). AIMS: The aim of this study was to see the results of ICT using cisplatin and Ifosfamide regimen in locally advanced unresectable SCCHN in terms of acute and chronic toxicity and response to treatment. MATERIALS AND METHODS: Patients with Stage III and IV, nonmetastatic SCCHN were enrolled in the study. They were given two cycles of ICT with cisplatin and Ifosfamide followed by CRT. RESULTS: After ICT, the overall response rate (ORR) was 75.0% at the primary site and 70.0% at the nodal site. ORR for combined primary and nodal disease was observed to be 67.5%. The complete response (CR) and partial response (PR) for combined primary and nodal site were seen in 4 (10.0%) and 23 (57.5%) patients. Of 32 patients who received CRT after ICT, CR was 53.1% and PR was 31.3%. Mucositis, skin reaction, and pharyngeal and laryngeal toxicities were the most common but tolerable. CONCLUSION: ICT with cisplatin and Ifosfamide gives comparable results to the standard paclitaxel, PF regimen. We conclude that this combination regimen for ICT is not only an economical alternative of taxol-based regimen but also well tolerated by the patients.
Investigating the heterogeneity of alkylating agents' efficacy and toxicity between sexes: A systematic review and meta-analysis of randomized trials comparing cyclophosphamide and ifosfamide (MAIAGE study).[Pubmed:28111876]
Pediatr Blood Cancer. 2017 Aug;64(8).
BACKGROUND: A marginal interaction between sex and the type of alkylating agent was observed for event-free survival in the Euro-EWING99-R1 randomized controlled trial (RCT) comparing cyclophosphamide and Ifosfamide in Ewing sarcoma. To further evaluate this interaction, we performed an individual patient data meta-analysis of RCTs assessing cyclophosphamide versus Ifosfamide in any type of cancer. METHODS: A literature search produced two more eligible RCTs (EICESS92 and IRS-IV). The endpoints were progression-free survival (PFS, main endpoint) and overall survival (OS). The hazard ratios (HRs) of the treatment-by-sex interaction and their 95% confidence interval (95% CI) were assessed using stratified multivariable Cox models. Heterogeneity of the interaction across age categories and trials was explored. We also assessed this interaction for severe acute toxicity using logistic models. RESULTS: The meta-analysis comprised 1,528 pediatric and young adult sarcoma patients from three RCTs: Euro-EWING99-R1 (n = 856), EICESS92 (n = 155), and IRS-IV (n = 517). There were 224 PFS events in Euro-EWING99-R1 and 200 in the validation set (EICESS92 + IRS-IV), and 171 and 154 deaths in each dataset, respectively. The estimated treatment-by-sex interaction for PFS in Euro-EWING99-R1 (HR = 1.73, 95% CI = 1.00-3.00) was not replicated in the validation set (HR = 0.97, 95% CI = 0.55-1.72), without heterogeneity across trials (P = 0.62). In the pooled analysis, the treatment-by-sex interaction was not significant (HR = 1.31, 95% CI = 0.89-1.95, P = 0.17), without heterogeneity across age categories (P = 0.88) and trials (P = 0.36). Similar results were observed for OS. No significant treatment-by-sex interaction was observed for leucopenia/neutropenia (P = 0.45), infection (P = 0.64), or renal toxicity (P = 0.20). CONCLUSION: Our meta-analysis did not confirm the hypothesis of a treatment-by-sex interaction on efficacy or toxicity outcomes.


