PreladenantAdenosine A2A receptor antagonist CAS# 377727-87-2 |
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- ANR 94
Catalog No.:BCC7815
CAS No.:634924-89-3
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 377727-87-2 | SDF | Download SDF |
| PubChem ID | 10117987 | Appearance | Powder |
| Formula | C25H29N9O3 | M.Wt | 503.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SCH-420814 | ||
| Solubility | DMSO : 5 mg/mL (9.93 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
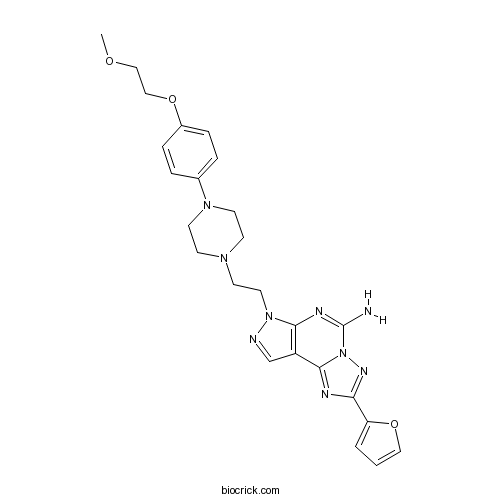
| SMILES | COCCOC1=CC=C(C=C1)N2CCN(CC2)CCN3C4=C(C=N3)C5=NC(=NN5C(=N4)N)C6=CC=CO6 | ||
| Standard InChIKey | DTYWJKSSUANMHD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H29N9O3/c1-35-15-16-36-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-23-20(17-27-33)24-28-22(21-3-2-14-37-21)30-34(24)25(26)29-23/h2-7,14,17H,8-13,15-16H2,1H3,(H2,26,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Preladenant (SCH 420814) is a potent and selective antagonist of adenosine A2A receptor. | |||||
| Targets | adenosine A2A receptor | |||||
| Cell experiment: [1] | |
| Cell lines | Primary actin-GFP microglia |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 1 μM, 15 min |
| Applications | Three-dimensional cell reconstructions from primary actin-GFP microglia grown in Matrigel were used to determine cell ramification (expressed as surface area-to-volume ratios) in response to preladenant treatment. Preladenant at concentration of 1 μM prevented the adenosine-induced process retraction in activated microglia. |
| Animal experiment : [2] | |
| Animal models | Male CD rats |
| Dosage form | Haloperidol (1 mg/kg s.c.) was administered to induce catalepsy in the rats. Preladenant was administered orally after the 30-min baseline measure, and catalepsy was retested 1 and 4 h after administration. |
| Application | Preladenant dose-dependently attenuated the cataleptic effects of haloperidol 1h [F(3,20) = 5.0, p < 0.01] and 4 h [F(3,20) = 9.8, p < 0.01] after dosing, with statistically significant effects at doses of 0.3 and 1 mg/kg at both time points. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Gyoneva S, Davalos D, Biswas D, et al. Systemic inflammation regulates microglial responses to tissue damage in vivo. Glia, 2014. [2] Hodgson R A, Bertorelli R, Varty G B, et al. Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2, 4-difluorophenyl]-1-piperazinyl] ethyl]-2-(2-furanyl)-7H-pyrazolo [4, 3-e][1, 2, 4] triazolo [1, 5-c] pyrimidin-5-amine] in rodent models of movement disorders and depression. Journal of Pharmacology and Experimental Therapeutics, 2009, 330(1): 294-303. | |

Preladenant Dilution Calculator

Preladenant Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9859 mL | 9.9293 mL | 19.8586 mL | 39.7172 mL | 49.6465 mL |
| 5 mM | 0.3972 mL | 1.9859 mL | 3.9717 mL | 7.9434 mL | 9.9293 mL |
| 10 mM | 0.1986 mL | 0.9929 mL | 1.9859 mL | 3.9717 mL | 4.9647 mL |
| 50 mM | 0.0397 mL | 0.1986 mL | 0.3972 mL | 0.7943 mL | 0.9929 mL |
| 100 mM | 0.0199 mL | 0.0993 mL | 0.1986 mL | 0.3972 mL | 0.4965 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Preladenant is a high selective antagonist of adenosine A2A receptor with Ki value of 1.1 nM [1].
Parkinson's Disease is characterized by the loss of dopaminergic neuronal projection. The patients with PD lost the capability to control their muscles. As an antagonist of adenosine A2A receptor, preladenant is a non-dopaminergic drug developed for the treatment of PD. This compound showed potency in Phase II clinical trials but failed in Phase III trials and so was discontinued. Preladenant is a methoxyethoxy derivative of the previously discovered A2A receptor antagonist SCH 58261 and has much higher selectivity for A2A receptors over A1 receptors [1 and 2].
Preladenant was obtained through modifying the phenethyl side chain of SCH 58261. It exerted higher binding affinity for both rat and human A2A receptors with Ki values of 2.5 and 1.1 nM, respectively. Preladenant also showed more than 1000-fold higher selectivity for A2A receptors over other adenosine receptors. The Ki values of preladenant for A1, A3 and A2B receptors are all above 1000 nM. Besides that, preladenant showed no significant affinity for a panel of 59 other enzymes, receptors and ion channels. In the cell assays, preladenant also inhibited the activity of both human and rat adenylate cyclase stimulated by CGS 21680 (an agonist of A2A receptor) with Kb values of 1.3 and 0.7 nM, respectively [1].
Preladenant also showed efficacies in animal models. In cynomolgus monkeys treated with MPTP, administration of preladenant at dose of both 1 and 3 mg/kg resulted in parkinsonian scores reduction. In a Dunnets post hoc test, the combination treatment of preladenant and L-Dopa significantly increased the locomotor activity by 80%. Besides that, preladenant was found to have anti- catalepsy effects and dose of 0.3 mg/kg and significantly reduce the hypolocomotion caused by CGS-21680 at dose of 0.1 mg/kg in rats models [2 and 3].
References:
[1] Neustadt B R, Hao J, Lindo N, et al. Potent, selective, and orally active adenosine A 2A receptor antagonists: arylpiperazine derivatives of pyrazolo [4, 3-e]-1, 2, 4-triazolo [1, 5-c] pyrimidines. Bioorganic & medicinal chemistry letters, 2007, 17(5): 1376-1380.
[2] Hodgson R A, Bedard P J, Varty G B, et al. Preladenant, a selective A 2A receptor antagonist, is active in primate models of movement disorders. Experimental neurology, 2010, 225(2): 384-390.
[3] Hodgson R A, Bertorelli R, Varty G B, et al. Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2, 4-difluorophenyl]-1-piperazinyl] ethyl]-2-(2-furanyl)-7H-pyrazolo [4, 3-e][1, 2, 4] triazolo [1, 5-c] pyrimidin-5-amine] in rodent models of movement disorders and depression. Journal of Pharmacology and Experimental Therapeutics, 2009, 330(1): 294-303.
- 2-Pentylfuran
Catalog No.:BCN3799
CAS No.:3777-69-3
- Zaprinast
Catalog No.:BCC6859
CAS No.:37762-06-4
- Enmein
Catalog No.:BCN3392
CAS No.:3776-39-4
- Iristectorin A
Catalog No.:BCN8221
CAS No.:37744-61-9
- Questin
Catalog No.:BCN7446
CAS No.:3774-64-9
- 2-Chloro-N6-cyclopentyladenosine
Catalog No.:BCC7161
CAS No.:37739-05-2
- Boc-Cha-OH
Catalog No.:BCC2661
CAS No.:37736-82-6
- Rhodojaponin V
Catalog No.:BCN2807
CAS No.:37720-86-8
- Totaradiol
Catalog No.:BCN5431
CAS No.:3772-56-3
- Dehydroabietinol
Catalog No.:BCN5430
CAS No.:3772-55-2
- SU 9516
Catalog No.:BCC2398
CAS No.:377090-84-1
- 8-Acetonyldihydrosanguinarine
Catalog No.:BCN5429
CAS No.:37687-34-6
- 7''-O-Methylsciadopitysin
Catalog No.:BCN4018
CAS No.:3778-25-4
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
- Boc-D-Pro-OH
Catalog No.:BCC3437
CAS No.:37784-17-1
- Cimigenol
Catalog No.:BCC8149
CAS No.:3779-59-7
- Betamethasone
Catalog No.:BCC4765
CAS No.:378-44-9
- ACDPP hydrochloride
Catalog No.:BCC7302
CAS No.:37804-11-8
- Phaseollidin
Catalog No.:BCN5432
CAS No.:37831-70-2
- Germacrene D
Catalog No.:BCN3851
CAS No.:37839-63-7
- Nepodin
Catalog No.:BCN6894
CAS No.:3785-24-8
- Ro 08-2750
Catalog No.:BCC7307
CAS No.:37854-59-4
- Scarlet 808
Catalog No.:BCC9139
CAS No.:3789-75-1
- Jolkinolide A
Catalog No.:BCN3771
CAS No.:37905-07-0
Synthesis of (3) H, (2) H4 , and (14) C-MK 3814 (preladenant).[Pubmed:28129428]
J Labelled Comp Radiopharm. 2017 Apr;60(4):194-199.
MK 3814 is a potent and selective antagonist of the A2a receptor. A2a receptor antagonists have the potential for the treatment of Parkinson disease. Three distinct isotopically labelled forms of MK 3814 were synthesized. [(3) H]MK 3814 was prepared for a preliminary absorption, distribution, metabolism, and excretion data (ADME) evaluation of the compound and [(14) C]MK 3814 for more definitive ADME work, including an absorption, metabolism, and excretion study in man. In addition, [(2) H4 ]MK 3814 was prepared as an internal standard for a liquid chromatography mass spectrometry bioanalytical method. This paper discusses the synthesis of 3 isotopically labelled forms of MK 3814.
In Vivo Evaluation of (11)C-Preladenant for PET Imaging of Adenosine A2A Receptors in the Conscious Monkey.[Pubmed:28062599]
J Nucl Med. 2017 May;58(5):762-767.
(11)C-Preladenant was developed as a novel PET ligand for the adenosine A2A receptors (A2ARs). The present study aimed to evaluate the suitability of (11)C-Preladenant PET for the quantification of striatal A2ARs and the assessment of A2AR occupancy in the conscious monkey brain. Methods:(11)C-Preladenant was intravenously injected into conscious monkeys (n = 4, 18 PET scans), and a 91-min dynamic scan was started. Arterial blood samples in combination with metabolite analysis were obtained during the scan to provide the input function for kinetic modeling. The distribution volume (VT) was obtained by kinetic modeling with a 2-tissue-compartment model. The simplified reference tissue model (SRTM) with selected reference regions (cerebellum, cingulate, parietal cortex, and occipital cortex) was tested to estimate the binding potential (BPND) in A2AR-rich regions. BPND obtained from the SRTM was compared with distribution volume ratio (DVR)-1. The effects of blood volume, blood delay, and scan duration on BPND and DVR-1 were investigated. VT and BPND were also obtained after preblocking with unlabeled Preladenant (1 mg/kg), A2AR-selective KW-6002 (0.5-1 mg/kg), and nonselective adenosine receptor antagonist caffeine (2.5-10 mg/kg). A2AR occupancy was studied with caffeine blockade. Results: Regional uptake of (11)C-Preladenant was consistent with the distribution of A2ARs in the monkey brain, with the highest uptake in the putamen, followed by the caudate, and the lowest uptake in the cerebellum. Tracer kinetics were well described by the 2-tissue-compartment model with a lower constraint on k4 to stabilize fits. The highest VT was observed in A2AR-rich regions ( approximately 5.8-7.4) and lowest value in the cerebellum ( approximately 1.3). BPND values estimated from the SRTM with different scan durations were comparable and were in agreement with DVR-1 ( approximately 4.3-5.3 in A2AR-rich regions). Preladenant preinjection decreased the tracer uptake in A2AR-rich regions to the level of the reference regions. Caffeine pretreatment reduced the tracer uptake in the striatum in a dose-dependent manner. Conclusion:(11)C-Preladenant PET is suitable for noninvasive quantification of A2ARs and assessment of A2AR occupancy in A2AR-rich regions in the monkey brain. SRTM using the cerebellum as the reference tissue is the applicable model for A2AR quantification.
Adjunctive preladenant: A placebo-controlled, dose-finding study in Japanese patients with Parkinson's disease.[Pubmed:27632893]
Parkinsonism Relat Disord. 2016 Nov;32:73-79.
BACKGROUND: Preladenant, an adenosine 2A antagonist, reduced daily OFF time when administered as adjunctive treatment in a previous phase 2 trial in non-Japanese Parkinson's disease (PD) patients on stable doses of levodopa. This study aimed to evaluate Preladenant as adjunctive therapy in Japanese patients with PD. METHODS: In this randomized, placebo-controlled, double-blind, 12-week, dose-ranging, phase 2 study, Japanese patients with moderate to severe PD on a stable regimen of levodopa were randomly assigned 1:1:1:1 to Preladenant 2 mg, 5 mg, or 10 mg BID or placebo. The primary efficacy end point was change from baseline to week 12 in mean OFF time, recorded using a PD diary. Safety and tolerability were also assessed. RESULTS: In total, 111 patients were randomly assigned to receive Preladenant 2 mg, and 113 each received Preladenant 5 mg, 10 mg, or placebo. In contrast to previous data, Preladenant in this study did not demonstrate statistically significant efficacy; the primary outcome was -0.7 h (P = 0.0564), -0.5 h (P = 0.1844), and -0.3 h (P = 0.3386), respectively, for Preladenant 2 mg, 5 mg, and 10 mg BID versus placebo. Overall, Preladenant was well tolerated, and the frequency of adverse events appeared to be dose related. CONCLUSIONS: In this phase 2 study, Preladenant used as adjunctive therapy in PD patients on stable doses of levodopa did not reduce mean OFF time; treatment was well tolerated at doses between 2 and 10 mg BID.
Initial Evaluation of an Adenosine A2A Receptor Ligand, (11)C-Preladenant, in Healthy Human Subjects.[Pubmed:28280214]
J Nucl Med. 2017 Sep;58(9):1464-1470.
(11)C-Preladenant is a selective antagonist for mapping of cerebral adenosine A2A receptors (A2ARs) by PET. This is a first-in-human study to examine the safety, radiation dosimetry, and brain imaging of (11)C-Preladenant in healthy human subjects. Methods: Dynamic (11)C-Preladenant PET scans (90 min) were obtained in 5 healthy male subjects. During the scan, arterial blood was sampled at various time intervals, and the fraction of the parent compound in plasma was determined. For anatomic coregistration, T1-weighted MRI was performed. The total distribution volume (VT) was estimated using 1- and 2-tissue-compartment models (1T and 2T, respectively). The distribution volume ratio (DVR) was calculated from VT of target and reference region and obtained with a noninvasive Logan graphical reference tissue method (t* = 30 min). The applicability of a shortened protocol as an alternative to the 90-min PET scan was investigated. Tracer biodistribution and dosimetry were determined in 3 healthy male subjects, using serial whole-body PET scans acquired over 2 h after (11)C-Preladenant injection. Results: There were no serious adverse events in any of the subjects throughout the study period. (11)C-preladenat readily entered the brain, with a peak uptake in the putamen and head of the caudate nucleus 30-40 min after tracer injection. Other brain regions showed rapid clearance of radioactivity. The regional distribution of (11)C-Preladenant was consistent with known A2AR densities in the brain. At pseudoequilibrium (reached at 40 min after injection), stable target-to-cerebellar cortex ratios of around 3.8-10.0 were obtained. The 2T fit better than the 1T in the low-density A2AR regions. In contrast, there were no significant differences between 1T and 2T in the high-A2AR-density regions. DVRs in the putamen and head of the caudate nucleus were around 3.8-10.3 when estimated using a Logan graphical reference tissue method with cerebellum as the reference region. PET scanning at 50 or 70 min can provide the stable DVR estimates within 10% or 5% differences at most, respectively. The radioactivity was mainly excreted through the hepatobiliary system after (11)C-Preladenant injection. As a result, the absorbed dose (muGy/MBq) was highest in the gallbladder wall (mean +/- SD, 17.0 +/- 2.5) and liver (11.7 +/- 2.1). The estimated effective dose for (11)C-Preladenant was 3.7 +/- 0.4 muSv/MBq. Conclusion: This initial evaluation indicated that (11)C-preladenat is suitable for imaging of A2ARs in the brain.


