ANR 94Adenosine A2A-R antagonist CAS# 634924-89-3 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 634924-89-3 | SDF | Download SDF |
| PubChem ID | 11805896 | Appearance | Powder |
| Formula | C9H13N5O | M.Wt | 207.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 100 mM in ethanol | ||
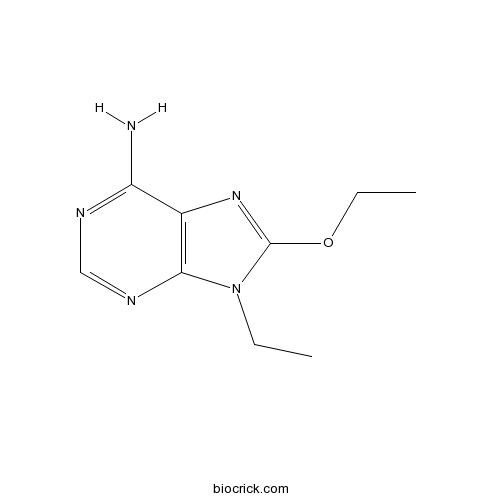
| Chemical Name | 8-ethoxy-9-ethylpurin-6-amine | ||
| SMILES | CCN1C2=C(C(=NC=N2)N)N=C1OCC | ||
| Standard InChIKey | QUGDTMONBLMLLD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H13N5O/c1-3-14-8-6(7(10)11-5-12-8)13-9(14)15-4-2/h5H,3-4H2,1-2H3,(H2,10,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Adenosine A2A receptor (AA2AR) antagonist (Ki values are 643 and 46 nM for rat and human AA2ARs respectively). Most active AA2AR antagonist for human receptors. Displays activity in the treatment of Parkinson's disease in vivo; improves parkinsonian motor deficits and tremors. Exhibits neuroprotective and anti-inflammatory effects. |

ANR 94 Dilution Calculator

ANR 94 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8256 mL | 24.1278 mL | 48.2556 mL | 96.5111 mL | 120.6389 mL |
| 5 mM | 0.9651 mL | 4.8256 mL | 9.6511 mL | 19.3022 mL | 24.1278 mL |
| 10 mM | 0.4826 mL | 2.4128 mL | 4.8256 mL | 9.6511 mL | 12.0639 mL |
| 50 mM | 0.0965 mL | 0.4826 mL | 0.9651 mL | 1.9302 mL | 2.4128 mL |
| 100 mM | 0.0483 mL | 0.2413 mL | 0.4826 mL | 0.9651 mL | 1.2064 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
8-ethoxy-9-ethyladenine (ANR 94) had been characterized in vitro as an adenosine receptor antagonist. Its chemical structure had been shown [1]. ANR 94 has shown high selectivity and affinity for the human adenosine A2A receptor subtype and high antiparkinsonian activity in unilaterally 6-hydroxydopamine (6-OHDA)-lesioned rats [2]. The Ki value of ANR 94 to the adenosine A2A receptor is 46 nM [1].
Adenosine is deeply involved in the control of motor behaviour and substantial evidences [1].
In Chinese hamster ovary (CHO) cells stably transfected with human recombinant adenosine receptors, ANR 94 was more selective than ANR 82 at the adenosine A2A receptor, with a Ki value of 46 nM [1]. Treatment with ANR 94 (0.5 mg/kg i.p. for 7 days) significantly prevented 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced degeneration of TH-positive cells (p < 0.0005) [2].
In rats, at a dose of 5 mg/kg i.p., ANR 94 did not modify spontaneous motility, whereas at higher doses (10 or 15 mg/kg), it induced hypermotility. ANR 94 at a dose of 1 mg/kg had a low efficacy on catalepsy, whereas 5 mg/kg was fully effective. In deeply cataleptic rats, ANR 94 at a dose of 5 mg/kg i.p. during the 90-min testing period significantly reversed the catalepsy induced by 0.2 mg/kg of haloperidol. From 30-60 min, the effect of ANR 94 was maximal. The anticataleptic effect of ANR 94 had a long duration of over 150 min. In 6-OHDA-lesioned rats, ANR 94 significantly increased the number of contralateral rotations induced by l-DOPA (3 mg/kg); this effect lasted up to 120-130 min [2].
References:
[1]. Annalisa Pinna, Rosaria Volpini, Gloria Cristalli, et al. New adenosine A2A receptor antagonists: Actions on Parkinson’s disease models. European Journal of Pharmacology, 2005, 512:157-164.
[2]. Annalisa Pinna, Elisabetta Tronci, Nicoletta Schintu, et al. A new ethyladenine antagonist of adenosine A2A receptors: Behavioral and biochemical characterization as an antiparkinsonian drug. Neuropharmacology, 2010, 58: 613-623.
- Lithospermoside
Catalog No.:BCN1270
CAS No.:63492-69-3
- WAY-100635 maleate salt
Catalog No.:BCC2054
CAS No.:634908-75-1
- TBPB
Catalog No.:BCC5517
CAS No.:634616-95-8
- Corollin
Catalog No.:BCC8754
CAS No.:63461-31-4
- 8-CPT-2Me-cAMP, sodium salt
Catalog No.:BCC7140
CAS No.:634207-53-7
- Alepterolic acid
Catalog No.:BCN4172
CAS No.:63399-38-2
- 3-Acetoxy-8(17),13E-labdadien-15-oic acid
Catalog No.:BCN1390
CAS No.:63399-37-1
- SJB2-043
Catalog No.:BCC1952
CAS No.:63388-44-3
- (+)-Isolariciresinol 9'-O-glucoside
Catalog No.:BCN7014
CAS No.:63358-12-3
- Bromethalin
Catalog No.:BCC5472
CAS No.:63333-35-7
- VU 10010
Catalog No.:BCC7577
CAS No.:633283-39-3
- Secoisolariciresinol monoglucoside
Catalog No.:BCN6990
CAS No.:63320-67-2
- Bilirubin
Catalog No.:BCN1047
CAS No.:635-65-4
- 4,2'-Dihydroxy-4'-methoxychalcone
Catalog No.:BCN7704
CAS No.:63529-06-6
- LY404039
Catalog No.:BCC4592
CAS No.:635318-11-5
- 24,25-Epoxydammar-20(21)-en-3-one
Catalog No.:BCN4173
CAS No.:63543-52-2
- 24,25-Dihydroxydammar-20-en-3-one
Catalog No.:BCN4174
CAS No.:63543-53-3
- Adrafinil
Catalog No.:BCC4166
CAS No.:63547-13-7
- Rebaudioside C
Catalog No.:BCN2404
CAS No.:63550-99-2
- 8-Benzoyl-5,7-dihydroxy-2,2-dimethylchromane
Catalog No.:BCN1389
CAS No.:63565-07-1
- Microhelenin C
Catalog No.:BCN7977
CAS No.:63569-07-3
- Pazopanib Hydrochloride
Catalog No.:BCC3708
CAS No.:635702-64-6
- Darunavir Ethanolate
Catalog No.:BCC5627
CAS No.:635728-49-3
- Foscarnet Sodium
Catalog No.:BCC4782
CAS No.:63585-09-1
New adenosine A2A receptor antagonists: actions on Parkinson's disease models.[Pubmed:15840400]
Eur J Pharmacol. 2005 Apr 11;512(2-3):157-64.
The 8-substituted 9-ethyladenine derivatives: 8-bromo-9-ethyladenine (ANR 82), 8-ethoxy- 9-ethyladenine (ANR 94), and 8-furyl-9-ethyladenine (ANR 152) have been characterized in vitro as adenosine receptor antagonists. Adenosine is deeply involved in the control of motor behaviour and substantial evidences indicate that adenosine A(2A) receptor antagonists improve motor deficits in animal models of Parkinson's disease. On this basis, the efficacy of ANR 82, ANR 94, and ANR 152 in rat models of Parkinson's disease was evaluated. All compounds tested reversed the catalepsy induced by haloperidol. However, in unilaterally 6-hydroxydopamine-lesioned rats, only ANR 94 and ANR 152 potentiated l-dihydroxy-phenylalanine (l-DOPA) effect on turning behaviour and induced contralateral turning behaviour in rats sensitised to l-DOPA. Taken together the results of this study indicate that some 8-substituted 9-ethyladenine derivatives ameliorate motor deficits in rat models of Parkinson's disease, suggesting a potential therapeutic role of these compounds.
A new ethyladenine antagonist of adenosine A(2A) receptors: behavioral and biochemical characterization as an antiparkinsonian drug.[Pubmed:19951715]
Neuropharmacology. 2010 Mar;58(3):613-23.
Adenosine A(2A) receptor antagonists have emerged as an attractive non-dopaminergic target in clinical trials aimed at evaluating improvement in motor deficits in Parkinson's disease (PD). Moreover, preclinical studies suggest that A(2A) receptor antagonists may slow the course of the underlying neurodegeneration of dopaminergic neurons. In this study, we evaluated the efficacy of the new adenosine A(2A) receptor antagonist 8-ethoxy-9-ethyladenine (ANR 94) in parkinsonian models of akinesia and tremor. In addition, induction of the immediate early gene zif-268, and neuroprotective and anti-inflammatory effects of ANR 94 were evaluated. ANR 94 was effective in reversing parkinsonian tremor induced by the administration of tacrine. ANR 94 also counteracted akinesia (stepping test) and sensorimotor deficits (vibrissae-elicited forelimb-placing test), as well as potentiating l-dopa-induced contralateral turning behavior in 6-hydroxydopamine (6-OHDA) lesion model of PD. Potentiation of motor behavior in 6-OHDA-lesioned rats was not associated with increased induction of the immediate early gene zif-268 in the striatum, suggesting that ANR 94 does not induce long-term plastic changes in this structure. Finally, in a subchronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD, ANR 94 protected nigrostriatal dopaminergic neurons from degeneration and counteracted neuroinflammatory processes by contrasting astroglial (glial fibrillary acidic protein, GFAP) and microglial (CD11b) activation. A(2A) receptor antagonism represents a uniquely realistic opportunity for improving PD treatment, since A(2A) receptor antagonists offer substantial symptomatic benefits and possibly disease-modifying activity. The characterization of ANR 94 may represent a further therapeutic opportunity for the treatment of PD with this new class of drugs.


