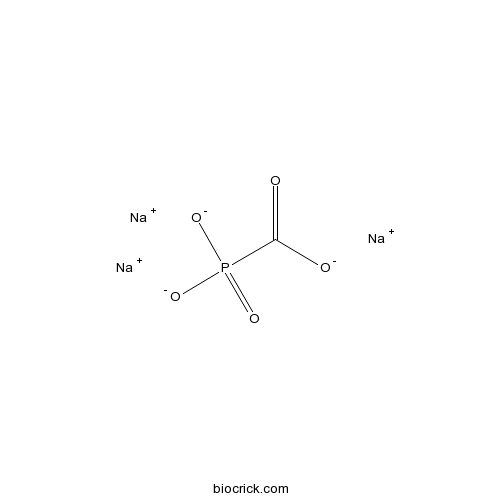
Foscarnet SodiumCAS# 63585-09-1 |
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63585-09-1 | SDF | Download SDF |
| PubChem ID | 44561 | Appearance | Powder |
| Formula | CNa3O5P | M.Wt | 191.95 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
| Chemical Name | trisodium;phosphonatoformate | ||
| SMILES | [Na+].[Na+].[Na+].[O-]C(=O)[P]([O-])([O-])=O | ||
| Standard InChIKey | DFHAXXVZCFXGOQ-UHFFFAOYSA-K | ||
| Standard InChI | InChI=1S/CH3O5P.3Na/c2-1(3)7(4,5)6;;;/h(H,2,3)(H2,4,5,6);;;/q;3*+1/p-3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Foscarnet Sodium Dilution Calculator

Foscarnet Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2097 mL | 26.0485 mL | 52.0969 mL | 104.1938 mL | 130.2423 mL |
| 5 mM | 1.0419 mL | 5.2097 mL | 10.4194 mL | 20.8388 mL | 26.0485 mL |
| 10 mM | 0.521 mL | 2.6048 mL | 5.2097 mL | 10.4194 mL | 13.0242 mL |
| 50 mM | 0.1042 mL | 0.521 mL | 1.0419 mL | 2.0839 mL | 2.6048 mL |
| 100 mM | 0.0521 mL | 0.2605 mL | 0.521 mL | 1.0419 mL | 1.3024 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Foscarnet Sodium inhibits viral RNA polymerases, reverse transcriptase, and DNA polymerases through noncompetitive inhibition with dNTPs.
- Darunavir Ethanolate
Catalog No.:BCC5627
CAS No.:635728-49-3
- Pazopanib Hydrochloride
Catalog No.:BCC3708
CAS No.:635702-64-6
- Microhelenin C
Catalog No.:BCN7977
CAS No.:63569-07-3
- 8-Benzoyl-5,7-dihydroxy-2,2-dimethylchromane
Catalog No.:BCN1389
CAS No.:63565-07-1
- Rebaudioside C
Catalog No.:BCN2404
CAS No.:63550-99-2
- Adrafinil
Catalog No.:BCC4166
CAS No.:63547-13-7
- 24,25-Dihydroxydammar-20-en-3-one
Catalog No.:BCN4174
CAS No.:63543-53-3
- 24,25-Epoxydammar-20(21)-en-3-one
Catalog No.:BCN4173
CAS No.:63543-52-2
- LY404039
Catalog No.:BCC4592
CAS No.:635318-11-5
- 4,2'-Dihydroxy-4'-methoxychalcone
Catalog No.:BCN7704
CAS No.:63529-06-6
- Bilirubin
Catalog No.:BCN1047
CAS No.:635-65-4
- ANR 94
Catalog No.:BCC7815
CAS No.:634924-89-3
- Terazosin
Catalog No.:BCC5162
CAS No.:63590-64-7
- 6-Hydroxydopamine hydrobromide
Catalog No.:BCC7403
CAS No.:636-00-0
- D-(+)-Maltose monohydrate
Catalog No.:BCN8423
CAS No.:6363-53-7
- DADLE
Catalog No.:BCC6064
CAS No.:63631-40-3
- Coniferyl ferulate
Catalog No.:BCN8543
CAS No.:63644-62-2
- 1,5-Bis(4-hydroxy-3-methoxyphenyl)penta-1,4-diene
Catalog No.:BCN1388
CAS No.:63644-68-8
- Gamma-Methoxyisoeugenol
Catalog No.:BCN3999
CAS No.:63644-71-3
- Z-D-Glu-OH
Catalog No.:BCC2775
CAS No.:63648-73-7
- Betaxolol HCl
Catalog No.:BCC4343
CAS No.:63659-19-8
- H-Lys(Z)-OBzl.HCl
Catalog No.:BCC2987
CAS No.:6366-70-7
- H-D-Asp-Obzl
Catalog No.:BCC2895
CAS No.:6367-42-6
- Nisoldipine
Catalog No.:BCC4809
CAS No.:63675-72-9
Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT.[Pubmed:20838386]
Bone Marrow Transplant. 2011 Jun;46(6):863-9.
Human herpesvirus-6 (HHV-6) is a major cause of limbic encephalitis with a dismal prognosis after allogeneic hematopoietic SCT (HSCT). A prospective, multicenter study was conducted to assess the safety and efficacy of preemptive therapy with Foscarnet Sodium (PFA) for the prevention of HHV-6 encephalitis. Plasma HHV-6 DNA was measured thrice weekly from day 7 until day 36 after umbilical cord blood transplantation (UCBT) or HSCT from HLA-haploidentical relatives. PFA, 90 mg/kg/day, was started when HHV-6 DNA exceeded 5 x 10(2) copies/mL. Mild and transient adverse events were associated with PFA in 7 of 8 patients. Twelve of 15 UCBT recipients became positive for HHV-6 DNAemia, defined by greater than 1 x 10(2) copies/mL of HHV-6 DNA in plasma. The virus exceeded 5 x 10(2) copies/mL in seven patients, whereas none of the five HLA-haploidentical HSCT recipients became positive. One patient developed mild limbic encephalitis just after initial PFA administration. Preemptive PFA therapy is safe, but as HHV-6 DNAemia can abruptly develop before neutrophil engraftment in UCBT recipients, prophylactic PFA administration from day 7 or earlier after UCBT may be needed.
[Foscarnet sodium for treatment in patients with severe chronic hepatitis B].[Pubmed:17125606]
Zhonghua Gan Zang Bing Za Zhi. 2006 Nov;14(11):814-6.
OBJECTIVE: To investigate the effectiveness of Foscarnet Sodium in the treatment of severe chronic hepatitis B. METHODS: Two hundred and eight patients were enrolled in a multicenter, double-blind, controlled study. The patients received Foscarnet Sodium (foscarnet group) or saline (control group) injections for 4 weeks, and were then followed for 24 weeks. RESULTS: HBV DNA negative rate was 12.8% in the foscarnet group and 7.1% in the control group at the end of treatment; and it was 5.5% and 3.0% at the end of the follow-up period respectively (P > 0.05). The rate of HBV DNA decrease of more than 2 log copies/ml was 53.2% in the foscarnet group and 16.2% in the control group at the end of treatment, and 23.9% and 8.1% (P < 0.01) respectively at the end of the follow-up period. The rate of HBV DNA < 10(5) copies/ml was 64.2% and 30.3% at week 4 in the two groups respectively, and 40.4% and 22.2% (P < 0.01) at the end of the follow-up period. HBeAg negative rate was 17.3% and 5.8% at the end of the treatment, and 22% and 5.4% at the end of the follow-up period (P < 0.01). The rate of HBeAg seroconversion was 12.7% and 3.7% at week 4, and 16.7% and 1.5% at the end of the follow-up period. Response rate was 60.6% and 21.2% at the end of week 4 (P < 0.05). CONCLUSION: Foscarnet Sodium injection has a good effect on severe chronic hepatitis B patients and it is safe to use on them.
Foscarnet, an inhibitor of the sodium-phosphate cotransporter NaPi-IIa, inhibits phosphorylation of glycogen synthase kinase-3beta by lithium in the rat kidney cortex.[Pubmed:27238574]
Drug Metab Pharmacokinet. 2016 Jun;31(3):256-9.
Lithium, which is used in the treatment of and prophylaxis for bipolar disease, inhibits glycogen synthase kinase-3beta (GSK3beta) by producing its phosphorylated form (p-GSK3beta). GSK3beta plays a role in apoptosis and some kinds of acute kidney injuries, and the formation of p-GSK3beta is considered to contribute to protection against acute kidney injury. We previously reported that the sodium-phosphate cotransporter NaPi-IIa (SLC34A1) mediated the reabsorption of lithium in the rat kidney. In the present study, the phosphorylation status of GSK3beta in the kidney cortex of rats administered lithium chloride and foscarnet, a typical inhibitor of NaPi-IIa, was examined using Western blotting. Under a 2-h infusion of lithium chloride, the plasma concentration of lithium was 1.06 mEq/l, and its renal clearance was calculated as 1.18 ml/min/kg, which was 29.6% of creatinine clearance. The abundance of p-GSK3beta in the kidney cortex was augmented by the administration of lithium. The simultaneous infusion of foscarnet increased the renal clearance of lithium and its ratio to creatinine clearance as well as the urinary excretion of phosphate. Foscarnet also inhibited the lithium-induced phosphorylation of GSK3beta. These results suggest that the reabsorption of lithium by NaPi-IIa triggers the phosphorylation of GSK3beta in the rat kidney cortex.
A comparison study on the clinical effects of foscarnet sodium injection and interferon on human immunodeficiency virus-infected patients complicated with herpes zoster.[Pubmed:26101481]
Pak J Med Sci. 2015 Mar-Apr;31(2):309-13.
OBJECTIVE: To compare the clinical effects of Foscarnet Sodium injection and interferon on human immunodeficiency virus (HIV)-infected patients complicated with herpes zoster. METHODS: Ninety HIV-infected patients complicated with herpes zoster were divided into a treatment group and a control group that were both treated routinely first. Then the control group and treatment group were administered with interferon and Foscarnet Sodium injection respectively for four consecutive weeks. RESULTS: After four weeks, the effective rates of the treatment and control groups were 95.6% and 80.0% respectively, which were significantly different (P < 0.05). The pain scores of the two groups were similar before treatment, but the scores of the treatment group were significantly lower than those of the control group two and four weeks after treatment (P < 0.05) as well as were significantly lower than those before treatment (P < 0.05). The numbers of CD4+ cells and the contents of IL-2 of both groups two and four weeks after treatment significantly exceeded those before treatment (P < 0.05), with significant inter-group differences also (P < 0.05). Two and four weeks after treatment, the treatment group scored significantly higher in physical activity, energy, sleep, social life and emotional reaction than the control group did (P < 0.05). CONCLUSIONS: HIV-infected patients are prone to being complicated with herpes zoster. Compared with interferon, Foscarnet Sodium injection better improves the clinical outcomes by effectively relieving pain and by regulating immune mediated inflammatory diseases, thus boosting the prognostic quality of life.


