SaquinavirHIV Protease Inhibitor CAS# 127779-20-8 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 127779-20-8 | SDF | Download SDF |
| PubChem ID | 441243 | Appearance | Powder |
| Formula | C38H50N6O5 | M.Wt | 670.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Invirase; Fortovase; Saguinavir;Ro 31-8959 | ||
| Solubility | DMSO : 100 mg/mL (149.07 mM; Need ultrasonic) | ||
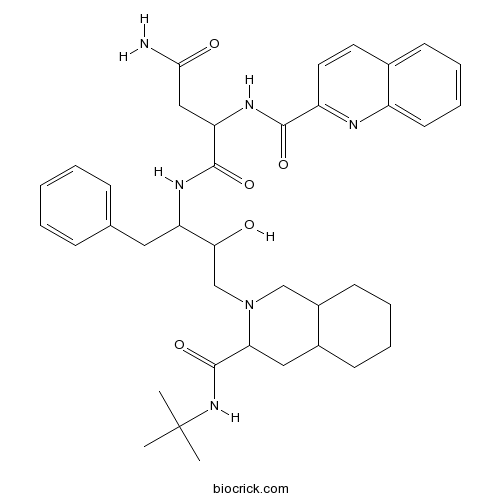
| Chemical Name | (2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarbamoyl)-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinolin-2-yl]-3-hydroxy-1-phenylbutan-2-yl]-2-(quinoline-2-carbonylamino)butanediamide | ||
| SMILES | CC(C)(C)NC(=O)C1CC2CCCCC2CN1CC(C(CC3=CC=CC=C3)NC(=O)C(CC(=O)N)NC(=O)C4=NC5=CC=CC=C5C=C4)O | ||
| Standard InChIKey | QWAXKHKRTORLEM-UGJKXSETSA-N | ||
| Standard InChI | InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Saquinavir(Ro 31-8959) is an HIV Protease inhibitor used in antiretroviral therapy.
IC50 Value:
Target: HIV Protease
Saquinavir is a protease inhibitor. Proteases are enzymes that cleave protein molecules into smaller fragments. HIV protease is vital for both viral replication within the cell and release of mature viral particles from an infected cell. Saquinavir binds to the active site of the viral protease and prevents cleavage of viral polyproteins, preventing maturation of the virus. Saquinavir inhibits both HIV-1 and HIV-2 proteases.Studies have also looked at Saquinavir as a possible anti-cancer agent. References: | |||||

Saquinavir Dilution Calculator

Saquinavir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4907 mL | 7.4533 mL | 14.9067 mL | 29.8134 mL | 37.2667 mL |
| 5 mM | 0.2981 mL | 1.4907 mL | 2.9813 mL | 5.9627 mL | 7.4533 mL |
| 10 mM | 0.1491 mL | 0.7453 mL | 1.4907 mL | 2.9813 mL | 3.7267 mL |
| 50 mM | 0.0298 mL | 0.1491 mL | 0.2981 mL | 0.5963 mL | 0.7453 mL |
| 100 mM | 0.0149 mL | 0.0745 mL | 0.1491 mL | 0.2981 mL | 0.3727 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Saquinavir is an HIV Protease Inhibitor used in antiretroviral therapy. Saquinavir inhibits both HIV-1 and HIV-2 proteases. Studies have also looked at Saquinavir as a possible anti-cancer agent.
- Dryocrassin ABBA
Catalog No.:BCN6276
CAS No.:12777-70-7
- 2'-O-Methylbroussonin A
Catalog No.:BCN7318
CAS No.:127757-13-5
- SKF 97541
Catalog No.:BCC6626
CAS No.:127729-35-5
- Radicicol
Catalog No.:BCC2131
CAS No.:12772-57-5
- (2R)-5,7-Dimethoxyflavanone
Catalog No.:BCN7806
CAS No.:1277188-85-8
- 9alpha,11-Dihydroxydrim-7-en-6-one
Catalog No.:BCN7225
CAS No.:127681-58-7
- PF-4989216
Catalog No.:BCC6468
CAS No.:1276553-09-3
- Cadherin Peptide, avian
Catalog No.:BCC1018
CAS No.:127650-08-2
- Oleficin
Catalog No.:BCN1848
CAS No.:12764-54-4
- Fananserin
Catalog No.:BCC7440
CAS No.:127625-29-0
- PF-3644022
Catalog No.:BCC6136
CAS No.:1276121-88-0
- HS-173
Catalog No.:BCC5363
CAS No.:1276110-06-5
- C-type natriuretic peptide (1-22) (human, rat, swine)
Catalog No.:BCC6033
CAS No.:127869-51-6
- CGP 37849
Catalog No.:BCC7078
CAS No.:127910-31-0
- CGP 39551
Catalog No.:BCC7053
CAS No.:127910-32-1
- 24(31)-Dehydrocarboxyacetylquercinic acid
Catalog No.:BCN1589
CAS No.:127970-62-1
- CU CPT 4a
Catalog No.:BCC6319
CAS No.:1279713-77-7
- Teucrin A
Catalog No.:BCC8259
CAS No.:12798-51-5
- Ursodiol
Catalog No.:BCC4945
CAS No.:128-13-2
- Pregnanolone
Catalog No.:BCC7736
CAS No.:128-20-1
- Sennoside B
Catalog No.:BCN1003
CAS No.:128-57-4
- Arvanil
Catalog No.:BCC7026
CAS No.:128007-31-8
- erythro-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1588
CAS No.:1280602-81-4
- Fmoc-D-Ser(tBu)-OH
Catalog No.:BCC3548
CAS No.:128107-47-1
Reduced Oral Bioavailability and Altered Pharmacokinetics of Saquinavir by Co-administration with Biochanin A in Rats.[Pubmed:27409329]
Drug Res (Stuttg). 2016 Sep;66(9):484-488.
The study was aim to assess the impact of biochanin A on the oral bioavailability and pharmacokinetics (PK) of Saquinavir (SQV), a substrate of P-glycoprotein (P-gp), in rats. 10 male rats were randomized into 2 groups of equal size, and administered orally 30 mg/kg SQV with or without 20 mg/kg biochanin A. The PK of SQV was assessed using non-compartmental analysis. Results revealed that the area under the plasma concentration-time curve of SQV from time zero to time infinity (AUC0-infinity) was reduced by 51.39% by biochanin A (P=0.038); while the apparent systemic clearance (CL/F) was increased by 87.62% (P=0.028). Double peak phenomenon was observed in the plasma SQV profiles. Biochanin A increased the first peak, yet decreased the second peak of plasma SQV levels. Our study demonstrates that biochanin A can significantly reduce SQV oral bioavailability and alter SQV PK profiles in rats. Findings in this study suggest a precaution in the clinic when SQV is administered with dietary/herbal supplements that contain biochanin A.
Identification of degradation products of saquinavir mesylate by ultra-high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry and its application to quality control.[Pubmed:28233930]
Rapid Commun Mass Spectrom. 2017 May 15;31(9):771-781.
RATIONALE: Saquinavir mesylate (SQM) is an antiviral drug used for the treatment of HIV infections. The identification and characterization of all degradation products are essential for achieving the quality in pharmaceutical product development and also for patient safety. METHODS: The drug was subjected to hydrolytic (HCl, NaOH and water), oxidative (H2 O2 ), photolytic (UV and fluorescence light) and thermal (dry heat) forced degradation conditions as per ICH guidelines. The best chromatographic separation of the drug and all degradation products (DPs) was achieved on a CSH-Phenyl Hexyl column (100 x 2.1 mm, 1.7 mum) with ammonium acetate (10 mM, pH 5.0) and methanol as mobile phase in gradient mode at a flow rate of 0.28 mL/min. RESULTS: Nine DPs were obtained under various forced degradation conditions. All the DPs were characterized by using ultra-high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC/ESI-QTOF MS/MS) and the degradation pathway of the drug was justified by mechanistic explanations. The main DPs were formed by amide hydrolysis, conversion into diastereomers, an N-oxide and dehydration as well as oxidation of the alcohol from the drug. The method was validated and can be used in a quality control (QC) laboratory to assure the quality of SQM in bulk and finished formulations. CONCLUSIONS: A simple UHPLC/photodiode array (PDA) method was developed and successfully transferred to UHPLC/ESI-Q-TOF MS/MS for the identification and characterization of DPs. Very interestingly, diastereomeric DPs were obtained and successfully resolved by the chromatographic method. Copyright (c) 2017 John Wiley & Sons, Ltd.
The HIV-protease inhibitor saquinavir reduces proliferation, invasion and clonogenicity in cervical cancer cell lines.[Pubmed:27698818]
Oncol Lett. 2016 Oct;12(4):2493-2500.
Innovative therapies in cervical cancer (CC) remain a priority. Recent data indicate that human immunodeficiency virus (HIV)-protease inhibitors used in highly active antiretroviral therapy can exert direct antitumor activities also in HIV-free preclinical and clinical models. The aim of the present study was to evaluate the antineoplastic effects of various HIV-protease inhibitors (indinavir, ritonavir and Saquinavir) on primary and established CC cell lines. Two CC cell lines established in our laboratory and four commercially available CC cell lines were treated with indinavir, ritonavir and Saquinavir at different concentrations and for different times. Proliferation, clonogenicity and radiosensitivity were evaluated by crystal violet staining. Proteasomal activities were assessed using a cell-based assay and immunoblotting. Cell cycle was analyzed by propidium iodide staining and flow cytometric analysis. Invasion was tested with Matrigel chambers. A t-test for paired samples was used for statistical analysis. In all cell lines, Saquinavir was more effective than ritonavir in reducing cell proliferation and inhibiting proteasomal activities (PSaquinavir concentrations required to modulate the proteasome activities were higher than those observed to be effective in inhibiting cell proliferation. In HeLa cells, Saquinavir was strongly effective in inhibiting cell invasion and clonogenicity (PSaquinavir did not contribute to increase the sensitivity of HeLa cells to X-rays. In conclusion, the present results demonstrate that Saquinavir is able to significantly reduce cell proliferation, cell invasion and clonogenicity in a proteasome-independent manner in in vitro models of CC, and suggest that Saquinavir could be a promising CC therapeutic agent.
Effects of resveratrol on P-glycoprotein and cytochrome P450 3A in vitro and on pharmacokinetics of oral saquinavir in rats.[Pubmed:27895462]
Drug Des Devel Ther. 2016 Nov 15;10:3699-3706.
BACKGROUND: The intestinal cytochrome P450 3A (CYP 3A) and P-glycoprotein (P-gp) present a barrier to the oral absorption of Saquinavir (SQV). Resveratrol (RESV) has been indicated to have modulatory effects on P-gp and CYP 3A. Therefore, this study was to investigate the effects of RESV on P-gp and CYP 3A activities in vitro and in vivo on oral SQV pharmacokinetics in rats. METHODS: In vitro, intestinal microsomes were used to evaluate RESV effect on CYP 3A-mediated metabolism of SQV; MDR1-expressing Madin-Darby canine kidney (MDCKII-MDR1) cells were employed to assess the impact of RESV on P-gp-mediated efflux of SQV. In vivo effects were studied using 10 rats randomly assigned to receive oral SQV (30 mg/kg) with or without RESV (20 mg/kg). Serial blood samples were obtained over the following 24 h. Concentrations of SQV in samples were ascertained using high-performance liquid chromatography-tandem mass spectrometry analysis. RESULTS: RESV (1-100 muM) enhanced residual SQV (% of control) in a dose-dependent manner after incubation with intestinal microsomes. RESV (1-100 muM) reduced the accumulation of SQV in MDCKII-MDR1 cells in a concentration-dependent manner. A double peaking phenomenon was observed in the plasma SQV profiles in rats. The first peak of plasma SQV concentration was increased, but the second peak was reduced by coadministration with RESV. The mean AUC0-infinity of SQV was slightly decreased, with no statistical significance probably due to the high individual variation. CONCLUSION: RESV can alter the plasma SQV concentration profiles, shorten the Tmax of SQV. RESV might also cause a slight decrease tendency in the SQV bioavailability in rats.


