OleficinCAS# 12764-54-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 12764-54-4 | SDF | Download SDF |
| PubChem ID | 54724553 | Appearance | Red powder |
| Formula | C34H47NO9 | M.Wt | 613.74 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
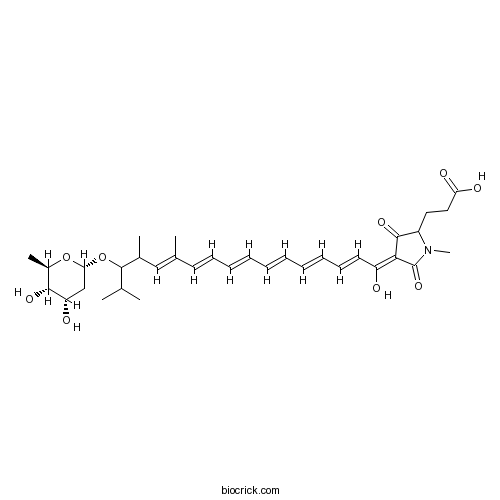
| Chemical Name | 3-[(4Z)-4-[(2E,4E,6E,8E,10E,12E)-15-[(2S,4S,5S,6R)-4,5-dihydroxy-6-methyloxan-2-yl]oxy-1-hydroxy-12,14,16-trimethylheptadeca-2,4,6,8,10,12-hexaenylidene]-1-methyl-3,5-dioxopyrrolidin-2-yl]propanoic acid | ||
| SMILES | CC1C(C(CC(O1)OC(C(C)C)C(C)C=C(C)C=CC=CC=CC=CC=CC(=C2C(=O)C(N(C2=O)C)CCC(=O)O)O)O)O | ||
| Standard InChIKey | UZDYIIINJYOXLQ-MKKOTRJMSA-N | ||
| Standard InChI | InChI=1S/C34H47NO9/c1-21(2)33(44-29-20-27(37)31(40)24(5)43-29)23(4)19-22(3)15-13-11-9-7-8-10-12-14-16-26(36)30-32(41)25(17-18-28(38)39)35(6)34(30)42/h7-16,19,21,23-25,27,29,31,33,36-37,40H,17-18,20H2,1-6H3,(H,38,39)/b8-7+,11-9+,12-10+,15-13+,16-14+,22-19+,30-26-/t23?,24-,25?,27+,29-,31-,33?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Oleficin, a polyene antibiotic, it is active against Grampositive bacteria and has no effect upon the growth of fungi and yeasts. 2. Oleficin has effects on mitochondrial functions can be explained on the basis of an increase of the inner membrane permeability as the consequence of the depletion of Mg2+ from mitochondria caused by the antibiotic. 3. Oleficin could as an ionophore of Mg2+ in isolated rat liver mitochondria, preferentially inhibit growth of the yeast Saccharomyces cerevisiae on non-fermentable substrates. |
| Targets | Sodium Channel | ATPase | Potassium Channel | Antifection |

Oleficin Dilution Calculator

Oleficin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6294 mL | 8.1468 mL | 16.2935 mL | 32.5871 mL | 40.7339 mL |
| 5 mM | 0.3259 mL | 1.6294 mL | 3.2587 mL | 6.5174 mL | 8.1468 mL |
| 10 mM | 0.1629 mL | 0.8147 mL | 1.6294 mL | 3.2587 mL | 4.0734 mL |
| 50 mM | 0.0326 mL | 0.1629 mL | 0.3259 mL | 0.6517 mL | 0.8147 mL |
| 100 mM | 0.0163 mL | 0.0815 mL | 0.1629 mL | 0.3259 mL | 0.4073 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fananserin
Catalog No.:BCC7440
CAS No.:127625-29-0
- PF-3644022
Catalog No.:BCC6136
CAS No.:1276121-88-0
- HS-173
Catalog No.:BCC5363
CAS No.:1276110-06-5
- CNX1351
Catalog No.:BCC6375
CAS No.:1276105-89-5
- VU 0360223
Catalog No.:BCC6159
CAS No.:1274859-33-4
- Conantokin-T
Catalog No.:BCC5977
CAS No.:127476-26-0
- Calystegine B1
Catalog No.:BCN1882
CAS No.:127414-86-2
- Calystegine B2
Catalog No.:BCN1879
CAS No.:127414-85-1
- Y-26763
Catalog No.:BCC7253
CAS No.:127408-31-5
- Y-27152
Catalog No.:BCC7254
CAS No.:127408-30-4
- Odoroside A
Catalog No.:BCC8224
CAS No.:12738-19-1
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Cadherin Peptide, avian
Catalog No.:BCC1018
CAS No.:127650-08-2
- PF-4989216
Catalog No.:BCC6468
CAS No.:1276553-09-3
- 9alpha,11-Dihydroxydrim-7-en-6-one
Catalog No.:BCN7225
CAS No.:127681-58-7
- (2R)-5,7-Dimethoxyflavanone
Catalog No.:BCN7806
CAS No.:1277188-85-8
- Radicicol
Catalog No.:BCC2131
CAS No.:12772-57-5
- SKF 97541
Catalog No.:BCC6626
CAS No.:127729-35-5
- 2'-O-Methylbroussonin A
Catalog No.:BCN7318
CAS No.:127757-13-5
- Dryocrassin ABBA
Catalog No.:BCN6276
CAS No.:12777-70-7
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- C-type natriuretic peptide (1-22) (human, rat, swine)
Catalog No.:BCC6033
CAS No.:127869-51-6
- CGP 37849
Catalog No.:BCC7078
CAS No.:127910-31-0
- CGP 39551
Catalog No.:BCC7053
CAS No.:127910-32-1
Ionophores and intact cells. II. Oleficin acts on mitochondria and induces disintegration of the mitochondrial genome in yeast Saccharomyces cerevisiae.[Pubmed:6818995]
Biochim Biophys Acta. 1982 Dec 30;721(4):349-56.
The non-macrolid polyene antibiotic Oleficin, which has been shown to function as an ionophore of Mg2+ in isolated rat liver mitochondria, preferentially inhibited growth of the yeast Saccharomyces cerevisiae on non-fermentable substrates. It uncoupled and inhibited respiration of intact cells and converted both growing and resting cells into respiration-deficient mutants. The mutants arose as a result of fragmentation of the mitochondrial genome. Another antibiotic known to be an ionophore of divalent cations, A23187, also selectively inhibited growth of the yeast on non-fermentable substrates, but did not produce the respiration-deficient mutants, neither antibiotic inhibited the energy-dependent uptake of divalent cations by yeast cells nor opened the plasma membrane for these cations. The results indicate that in Saccharomyces cerevisiae both Oleficin and A23187 preferentially affected the mitochondrial membrane without acting as ionophores in the plasma membrane.
Interaction of oleficin with the inner membrane of rat liver mitochondria.[Pubmed:6448831]
J Antibiot (Tokyo). 1980 May;33(5):494-500.
The effects of Oleficin, a polyene antibiotic of the nonmacrolide type, on isolated rat liver mitochondria were studied. Oleficin at a concentration of about 10 nmoles/mg protein increases both the rate of state 4 respiration and the "basal" ATPase activity of mitochondria. In contrast to this it inhibits the rate of both state 3 and uncoupled respiration and the DNP-stimulated ATPase activity. These inhibitions can be prevented by low concentrations (2 approximately 5 mM) of magnesium ions. Oleficin induces a high amplitude swelling of non-respiring mitochondria in the isoosmotic nitrate and chloride solutions of K+, Na+, Tris+, Tea+ or Mg2+. In contrast to that it does not induce swelling of mitochondria treated with ruthenium red in isoosmotic calcium acetate. Indirect evidence suggests that Oleficin increases also the proton permeability of the inner membrane. The swelling observed in the isoosmotic solutions of monovalent cations can be prevented by low concentration (2 approximately 5 mM) of Mg2+. In the presence of the antibiotic Mg2+ and Ca2+ but not K+ and Na+, are transferred from an aqueous phase into a butanol-toluene bulk phase. Oleficin depletes Mg2+ and Ca2+ from mitochondria in a concentration dependent manner. Complete depletion of Mg2+ occurs only in the presence of EDTA, while that of Ca2+ does not need the chelator. It is concluded that the effects of Oleficin on mitochondrial functions can be explained on the basis of an increase of the inner membrane permeability as the consequence of the depletion of Mg2+ from mitochondria caused by the antibiotic.


