Odoroside ACAS# 12738-19-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 12738-19-1 | SDF | Download SDF |
| PubChem ID | 44425145 | Appearance | Powder |
| Formula | C30H46O7 | M.Wt | 518.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
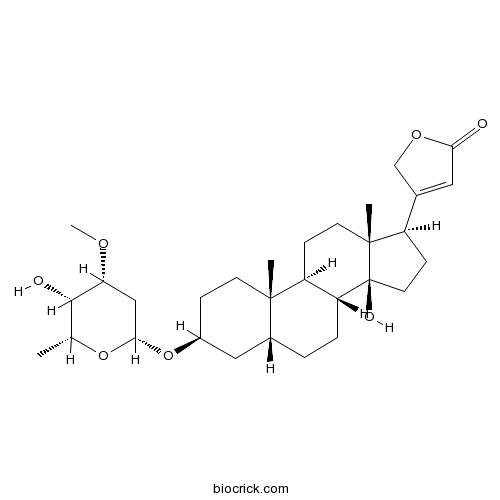
| Chemical Name | 3-[(3S,5R,8R,9S,10S,13R,14S,17R)-14-hydroxy-3-[(2R,4R,5S,6R)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | ||
| SMILES | CC1C(C(CC(O1)OC2CCC3(C(C2)CCC4C3CCC5(C4(CCC5C6=CC(=O)OC6)O)C)C)OC)O | ||
| Standard InChIKey | YBZZSZQZDODUAA-FNFYTULRSA-N | ||
| Standard InChI | InChI=1S/C30H46O7/c1-17-27(32)24(34-4)15-26(36-17)37-20-7-10-28(2)19(14-20)5-6-23-22(28)8-11-29(3)21(9-12-30(23,29)33)18-13-25(31)35-16-18/h13,17,19-24,26-27,32-33H,5-12,14-16H2,1-4H3/t17-,19-,20+,21-,22+,23-,24-,26+,27+,28+,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Odoroside A Dilution Calculator

Odoroside A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9279 mL | 9.6395 mL | 19.279 mL | 38.5579 mL | 48.1974 mL |
| 5 mM | 0.3856 mL | 1.9279 mL | 3.8558 mL | 7.7116 mL | 9.6395 mL |
| 10 mM | 0.1928 mL | 0.9639 mL | 1.9279 mL | 3.8558 mL | 4.8197 mL |
| 50 mM | 0.0386 mL | 0.1928 mL | 0.3856 mL | 0.7712 mL | 0.9639 mL |
| 100 mM | 0.0193 mL | 0.0964 mL | 0.1928 mL | 0.3856 mL | 0.482 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Coclauril
Catalog No.:BCN6150
CAS No.:127350-68-9
- Rebaudioside G
Catalog No.:BCN7860
CAS No.:127345-21-5
- 2-Chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride
Catalog No.:BCC8569
CAS No.:127337-60-4
- YLF-466D
Catalog No.:BCC4086
CAS No.:1273323-67-3
- PACAP 1-27
Catalog No.:BCC5726
CAS No.:127317-03-7
- Zamifenacin fumarate
Catalog No.:BCC7418
CAS No.:127308-98-9
- BRL 37344, sodium salt
Catalog No.:BCC6860
CAS No.:127299-93-8
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Sitafloxacin
Catalog No.:BCC5164
CAS No.:127254-12-0
- Intermedin B
Catalog No.:BCN7317
CAS No.:127214-87-3
- Bisacurone C
Catalog No.:BCN7316
CAS No.:127214-86-2
- Y-27152
Catalog No.:BCC7254
CAS No.:127408-30-4
- Y-26763
Catalog No.:BCC7253
CAS No.:127408-31-5
- Calystegine B2
Catalog No.:BCN1879
CAS No.:127414-85-1
- Calystegine B1
Catalog No.:BCN1882
CAS No.:127414-86-2
- Conantokin-T
Catalog No.:BCC5977
CAS No.:127476-26-0
- VU 0360223
Catalog No.:BCC6159
CAS No.:1274859-33-4
- CNX1351
Catalog No.:BCC6375
CAS No.:1276105-89-5
- HS-173
Catalog No.:BCC5363
CAS No.:1276110-06-5
- PF-3644022
Catalog No.:BCC6136
CAS No.:1276121-88-0
- Fananserin
Catalog No.:BCC7440
CAS No.:127625-29-0
- Oleficin
Catalog No.:BCN1848
CAS No.:12764-54-4
- Cadherin Peptide, avian
Catalog No.:BCC1018
CAS No.:127650-08-2
Odoroside A and ouabain inhibit Na+/K+-ATPase and prevent NF-kappaB-inducible protein expression by blocking Na+-dependent amino acid transport.[Pubmed:19559678]
Biochem Pharmacol. 2009 Nov 1;78(9):1157-66.
Inflammatory cytokines, such as tumor necrosis factor (TNF)-alpha and interleukin-1 (IL-1), trigger the activation of transcription factor NF-kappaB that induces the expression of a variety of genes, including intercellular adhesion molecule (ICAM)-1. Odoroside A [3beta-O-(beta-D-diginosyl)-14-hydroxy-5beta,14beta-card-20(22)-enolide] was found to inhibit the cell-surface expression of ICAM-1 induced by TNF-alpha and IL-1 at comparable concentrations in human lung carcinoma A549 cells. In this study, the molecular mechanism underlying the inhibition of TNF-alpha-induced cell-surface ICAM-1 expression by Odoroside A together with the specific Na(+)/K(+)-ATPase inhibitor ouabain was further investigated. Odoroside A and ouabain neither prevented IkappaBalpha degradation nor NF-kappaB translocation to the nucleus upon TNF-alpha stimulation. While Odoroside A and ouabain had no inhibitory effect on the induction of ICAM-1 mRNA, they inhibited the TNF-alpha-induced ICAM-1 expression at the protein level. Consistent with these results, Odoroside A and ouabain potently reduced de novo protein synthesis, largely due to its ability to block Na(+)-dependent transport of amino acids across the plasma membrane, but not to interfering with the translation machinery. As a direct molecular target, Odoroside A was found to inhibit the ATP-hydrolyzing activity of Na(+)/K(+)-ATPase as potently as ouabain. These results clearly demonstrate that Odoroside A and ouabain prevent NF-kappaB-inducible protein expression by blocking the Na(+)-dependent amino acid transport.


