BRL 37344, sodium saltβ3-adrenoceptor agonist,potent and selective CAS# 127299-93-8 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 127299-93-8 | SDF | Download SDF |
| PubChem ID | 16219010 | Appearance | Powder |
| Formula | C19H21NO4ClNa | M.Wt | 385.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
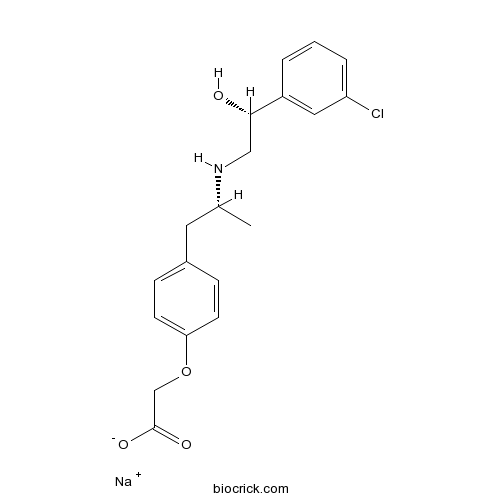
| Chemical Name | sodium;2-[4-[(2R)-2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]phenoxy]acetate | ||
| SMILES | CC(CC1=CC=C(C=C1)OCC(=O)[O-])NCC(C2=CC(=CC=C2)Cl)O.[Na+] | ||
| Standard InChIKey | SNJIJYKMYQRHRC-WJKBNZMCSA-M | ||
| Standard InChI | InChI=1S/C19H22ClNO4.Na/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24;/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24);/q;+1/p-1/t13-,18+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective β3 adrenoceptor agonist (Ki values are 287, 1750 and 1120 nM for β3, β1 and β2 receptors respectively). Also available as part of the β-Adrenoceptor Agonist. |

BRL 37344, sodium salt Dilution Calculator

BRL 37344, sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5919 mL | 12.9594 mL | 25.9188 mL | 51.8376 mL | 64.7971 mL |
| 5 mM | 0.5184 mL | 2.5919 mL | 5.1838 mL | 10.3675 mL | 12.9594 mL |
| 10 mM | 0.2592 mL | 1.2959 mL | 2.5919 mL | 5.1838 mL | 6.4797 mL |
| 50 mM | 0.0518 mL | 0.2592 mL | 0.5184 mL | 1.0368 mL | 1.2959 mL |
| 100 mM | 0.0259 mL | 0.1296 mL | 0.2592 mL | 0.5184 mL | 0.648 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BRL 37344, sodium salt is a potent and selective agonist of β3 adrenoceptor with Ki values of 287, 1750 and 1120 nM for β3, β1 and β2 receptors, respectively [1].
β3 adrenoceptor is exists mainly in adipose tissue and plays an important role in the regulation of thermogenesis and lipolysis.
BRL 37344, sodium salt is a potent and selective β3 adrenoceptor agonist. In isolated rat brown and white adipocytes, BRL 37344 significantly stimulated lipolysis with EC50 values of 5 and 56 nM in brown adipocytes and white adipocytes, respectively [1]. In the isolated guinea pig heart, BRL 37344 (10-8-10-5 M) increased heart contractility (dP/dt) and coronary flow (CF). However, BRL 37344 (10-8 M) completely inhibited isoprenaline-induced increase in contractility, which suggested that BRL 37344 display β1-antagonistic properties [2].
In fasted rabbits, BRL 37344 significantly increased plasma nonesterified fatty acids (NEFA) levels through β3-adrenoceptor. However, BRL 37344 had no effect on plasma glucose levels [3]. In mice, BRL 37344 increased circulating transaminase levels through activation of β3-adrenoceptor [4].
References:
[1]. Simard PM, Atgié C, Mauriège P, et al. Comparison of the lipolytic effects of norepinephrine and BRL 37344 in rat brown and white adipocytes. Obes Res, 1994, 2(5): 424-431.
[2]. Kozlovski VI, Chlopicki S, Gryglewski RJ. Effects of two beta3-agonists, CGP 12177A and BRL 37344, on coronary flow and contractility in isolated guinea pig heart. J Cardiovasc Pharmacol, 2003, 41(5): 706-713.
[3]. Reverte M, Rivas-Cabañero L. Effects of the beta 3-adrenoceptor agonist BRL 37344 on lipomobilization and plasma glucose levels in conscious fasted rabbits. Can J Physiol Pharmacol, 1996, 74(3): 251-256.
[4]. Kasahara H, Muto S, Motokawa Y, et al. β3-adrenoceptor-mediated increased circulating transaminase levels in mice treated with its agonist BRL 37344. J Toxicol Sci, 2010, 35(5): 779-784.
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Sitafloxacin
Catalog No.:BCC5164
CAS No.:127254-12-0
- Intermedin B
Catalog No.:BCN7317
CAS No.:127214-87-3
- Bisacurone C
Catalog No.:BCN7316
CAS No.:127214-86-2
- (3S,4S)-3-(Boc-amino)-4-methylpyrrolidine
Catalog No.:BCC4015
CAS No.:127199-54-6
- KN-62
Catalog No.:BCC3602
CAS No.:127191-97-3
- 7-(2'-Deoxyadenosin-N6-yl)aristolactam I
Catalog No.:BCN2559
CAS No.:127191-86-0
- 4-O-Demethylkadsurenin D
Catalog No.:BCN6649
CAS No.:127179-70-8
- MI-2
Catalog No.:BCC1746
CAS No.:1271738-62-5
- MI-3
Catalog No.:BCC1747
CAS No.:1271738-59-0
- BMS-911543
Catalog No.:BCC2204
CAS No.:1271022-90-2
- Glyceryl hexacosanoate
Catalog No.:BCC8991
CAS No.:127098-14-0
- Zamifenacin fumarate
Catalog No.:BCC7418
CAS No.:127308-98-9
- PACAP 1-27
Catalog No.:BCC5726
CAS No.:127317-03-7
- YLF-466D
Catalog No.:BCC4086
CAS No.:1273323-67-3
- 2-Chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride
Catalog No.:BCC8569
CAS No.:127337-60-4
- Rebaudioside G
Catalog No.:BCN7860
CAS No.:127345-21-5
- Coclauril
Catalog No.:BCN6150
CAS No.:127350-68-9
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Odoroside A
Catalog No.:BCC8224
CAS No.:12738-19-1
- Y-27152
Catalog No.:BCC7254
CAS No.:127408-30-4
- Y-26763
Catalog No.:BCC7253
CAS No.:127408-31-5
- Calystegine B2
Catalog No.:BCN1879
CAS No.:127414-85-1
- Calystegine B1
Catalog No.:BCN1882
CAS No.:127414-86-2
BRL37344, but not CGP12177, stimulates fuel oxidation by soleus muscle in vitro.[Pubmed:11011029]
Eur J Pharmacol. 2000 Oct 6;406(1):33-40.
The beta(3)-adrenoceptor agonist, (RR+SS)-(+/-)-4-[2-)2-)3-chlorophenyl)-2-hydroxyethyl)amino)propyl]ph enoxyacetate (BRL37344), stimulated fuel utilisation by isolated mouse soleus muscle at concentrations 10- to 100-fold lower than those required to stimulate lipolysis in brown adipocytes. At 1x10(-10) M BRL37344, uptake and phosphorylation of 2-deoxyglucose was increased (40%), as was glucose-oxidation (50%), palmitate-oxidation (70%) and oxidation of [2-14C]pyruvate (2-fold), indicating stimulation of tricarboxylic acid cycle reactions. Oxidation of [1-14C]pyruvate was unaffected, indicating no stimulation of pyruvate dehydrogenase activity. Other beta(3)-adrenoceptor agonists, disodium(RR)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]propyl]- 1,3-benzodioxazole-2,2-dicarboxylate (CL316,243, 1x10(-7) M) and (S)-4- inverted question mark2-[2-hydroxy-3-(4-hydroxyphenoxy)propylamino]ethyl inverted question markpheno xymeth ylcyclohexylphosphiric acid lithium salt (SB226552, 1x10(-9) M), achieved similar stimulation of 2-deoxyglucose uptake and phosphorylation but (+/-)-4-(3-t-butylamino-2-hydroxypropoxy)benzimidazol-2-one (CGP12177A) had no effect. The inhibitor of protein kinase A, H-89 (isoquinolinesulfonamide), had little effect on the stimulation of pyruvate-oxidation by BRL37344, while the specific inhibitor of protein kinase C, bisindolylmaleimide IX, reduced the stimulated rate to slightly below basal values. We consider that these responses provide evidence of the presence of a novel beta-adrenoceptor in skeletal muscle, which we have termed beta(skel)-adrenoceptor.
The selectivity in vitro of the stereoisomers of the beta-3 adrenoceptor agonist BRL 37344.[Pubmed:8613923]
J Pharmacol Exp Ther. 1996 Apr;277(1):22-7.
The stimulation by BRL 37344 of lipolysis in rat adipose tissues, and of relaxation of the rat distal colon, is mediated by the beta-3 adrenoceptor. The stereochemical requirements of the beta-3 adrenoceptor are poorly understood. The activities of the four stereoisomers of BRL 37344 (i.e., two pairs of diastereoisomers) on three beta-3 adrenoceptor-mediated responses (brown and white adipose tissue lipolysis and relaxation of distal colon) have been determined and compared with those responses mediated by beta-1 adrenoceptors (increase in atrial rate) and beta-2 adrenoceptors (uterine relaxation). The potency order for the stereoisomers (RR>RS=SR>>SS) was the same for all tissues, regardless of whether the response was mediated by beta-1, beta-2 or beta-3 adrenoceptors. These results indicate that both chiral centers are determinants of agonist potency at all three subtypes of the beta adrenoceptor. Furthermore, agonist activity at beta-1, beta-2 and beta-3 adrenoceptors resides predominantly with the RR enantiomer. Finally, the RR enantiomer of BRL 37344 was a more potent agonist in brown adipocytes (EC50 = 3.3 +/- 0.8 nM) than in white adipocytes (EC50 = 5.7 +/- 0.9 nM) or colon (EC50 = 27.5 +/- 7.7 nM).
Function and regulation of the beta 3-adrenoceptor.[Pubmed:8979772]
Trends Pharmacol Sci. 1996 Oct;17(10):373-81.
The cloning, sequencing and expression in model systems of the previously unidentified beta 3-adrenoceptor recently led to an extensive functional characterization. Ligand binding and adenylate cyclase activation studies helped define a specific profile that is quite distinct from that of the beta 1- and beta 2-adrenoceptors, but strongly reminiscent of most of the 'atypical' beta-adrenoceptor-mediated responses reported in earlier pharmacological studies. More recently, a naturally occurring variation in the human beta 3-adrenoceptor has been correlated with hereditary obesity and with increased dynamic capacity to add on weight and develop non-insulin dependent diabetes in Western obese patients. Donny Strosberg and France Pietri-Rouxel describe how results now provide a consistent picture of an important role for the human beta 3-adrenoceptor in the regulation of lipid metabolism and as an obvious target for drugs to treat some forms of obesity and diabetes.
Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs.[Pubmed:6325935]
Nature. 1984 May 10-16;309(5964):163-5.
Recent studies suggest that thermogenesis in brown adipose tissue has an important role in the regulation of energy balance. Thermogenesis is effected by noradrenaline released from sympathetic nerve endings; the noradrenaline stimulates beta-adrenoceptors, causing lipolysis, and the released fatty acids then promote the uncoupling of oxidative phosphorylation from electron transport. It has been widely accepted that mammalian beta-adrenoceptors exist as two subtypes, beta 1 and beta 2, and rat brown adipocyte beta-adrenoceptors have been classed as beta 1 or as a mixed beta 1/beta 2 population. The beta 1 subtype predominates in atria, whereas the beta 2 subtype predominates in trachea. However, we have now found a novel group of beta-adrenoceptor agonists that selectively stimulate lipolysis in brown adipocytes. In contrast, isoprenaline, fenoterol and salbutamol are less potent as stimulants of lipolysis than as stimulants of atrial rate or tracheal relaxation. Therefore, beta-adrenoceptors in rat brown adipocytes are of neither the beta 1 nor beta 2 subtypes. Compounds that selectively stimulate brown adipocyte beta-adrenoceptors should have potential as thermogenic anti-obesity agents and this has been demonstrated with BRL 26830A , BRL 33725A and BRL 35135A .


