KN-62CaM kinase II inhibitor CAS# 127191-97-3 |
- KN-93
Catalog No.:BCC1683
CAS No.:139298-40-1
- A 484954
Catalog No.:BCC6203
CAS No.:142557-61-7
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 127191-97-3 | SDF | Download SDF |
| PubChem ID | 3838 | Appearance | Powder |
| Formula | C38H35N5O6S2 | M.Wt | 721.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (138.53 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
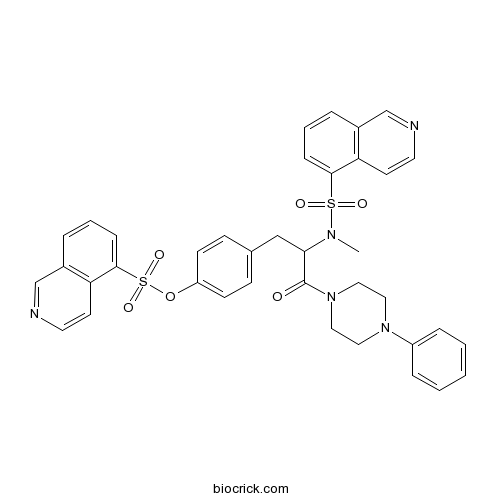
| Chemical Name | [4-[2-[isoquinolin-5-ylsulfonyl(methyl)amino]-3-oxo-3-(4-phenylpiperazin-1-yl)propyl]phenyl] isoquinoline-5-sulfonate | ||
| SMILES | CN(C(CC1=CC=C(C=C1)OS(=O)(=O)C2=CC=CC3=C2C=CN=C3)C(=O)N4CCN(CC4)C5=CC=CC=C5)S(=O)(=O)C6=CC=CC7=C6C=CN=C7 | ||
| Standard InChIKey | RJVLFQBBRSMWHX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C38H35N5O6S2/c1-41(50(45,46)36-11-5-7-29-26-39-19-17-33(29)36)35(38(44)43-23-21-42(22-24-43)31-9-3-2-4-10-31)25-28-13-15-32(16-14-28)49-51(47,48)37-12-6-8-30-27-40-20-18-34(30)37/h2-20,26-27,35H,21-25H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, cell-permeable inhibitor of CaM kinase II (IC50 = 0.9 μM). Binds directly to the calmodulin binding site of the enzyme. Potent non-competitive antagonist at the P2X7 receptor (IC50 = 15 nM). |

KN-62 Dilution Calculator

KN-62 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3852 mL | 6.9262 mL | 13.8523 mL | 27.7047 mL | 34.6308 mL |
| 5 mM | 0.277 mL | 1.3852 mL | 2.7705 mL | 5.5409 mL | 6.9262 mL |
| 10 mM | 0.1385 mL | 0.6926 mL | 1.3852 mL | 2.7705 mL | 3.4631 mL |
| 50 mM | 0.0277 mL | 0.1385 mL | 0.277 mL | 0.5541 mL | 0.6926 mL |
| 100 mM | 0.0139 mL | 0.0693 mL | 0.1385 mL | 0.277 mL | 0.3463 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KN-62, 1-[N,O-bis-(5-isoquinolinesulphonyl)-N-methyl-L-tyrosy]-4-phenylpiperazine is a highly selective inhibitor of the calcium dependent calmodulin protein kinase II (CAMKII), which binds to the calmodulin binding site of CAMKII and consequently does not inhibit other classes of calmodulin-sensitive kinases. In previous studies, KN-62 exhibits inhibitory effects on the regulated secretion of insulin by hetero-junction with intrinsic thin-layer (HIT) cells and the cholecystokinin secretion from stanniocalcin-1 (STC-1) enteroendocrine cells due to a block of Ca2+ influx through L-type Ca2+ channels in the olasma membrane. It also inhibits both insulin- and hypoxia-stimulated glucose transport activity by 46% and 40% respectively in skeletal muscle.
Reference
Joseph T. Brozinick, Jr., Thomas H. Reynolds, David Dean, Gregory Cartee and Samuel W. Cushman. 1-[N,O-bis-(5-isoquinolinesulphonyl)-N-methyl-L-tyrosy]-4-phenylpiperazine (KN-62), an inhibitor of calcium-dependent camodulin protein kinase II, inhibits both insulin- and hypoxia-stimulated glucose transport in skeletal muscle. Biochem. J. (1999) 339, 533-540
Erik S. Schweitzer, Michael J. Sanderson and C. G. Wasterlain. Inhibition of regulated catecholamine secretion from PC12 cells by the Ca2+/calmodulin kinase II inhibitor KN-62. Journal of Cell Science 108. 2619-2628 (1995)
- 7-(2'-Deoxyadenosin-N6-yl)aristolactam I
Catalog No.:BCN2559
CAS No.:127191-86-0
- 4-O-Demethylkadsurenin D
Catalog No.:BCN6649
CAS No.:127179-70-8
- MI-2
Catalog No.:BCC1746
CAS No.:1271738-62-5
- MI-3
Catalog No.:BCC1747
CAS No.:1271738-59-0
- BMS-911543
Catalog No.:BCC2204
CAS No.:1271022-90-2
- Glyceryl hexacosanoate
Catalog No.:BCC8991
CAS No.:127098-14-0
- Sulfo-NHS-LC-Biotin
Catalog No.:BCC3578
CAS No.:127062-22-0
- CGP 42112
Catalog No.:BCC5921
CAS No.:127060-75-7
- 4,9-Dimethoxycanthin-6-one
Catalog No.:BCN3107
CAS No.:1270001-72-3
- Beta-Pinene
Catalog No.:BCC8302
CAS No.:127-91-3
- Sulfamerazine
Catalog No.:BCC4854
CAS No.:127-79-7
- Sulfisoxazole
Catalog No.:BCC4860
CAS No.:127-69-5
- (3S,4S)-3-(Boc-amino)-4-methylpyrrolidine
Catalog No.:BCC4015
CAS No.:127199-54-6
- Bisacurone C
Catalog No.:BCN7316
CAS No.:127214-86-2
- Intermedin B
Catalog No.:BCN7317
CAS No.:127214-87-3
- Sitafloxacin
Catalog No.:BCC5164
CAS No.:127254-12-0
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- BRL 37344, sodium salt
Catalog No.:BCC6860
CAS No.:127299-93-8
- Zamifenacin fumarate
Catalog No.:BCC7418
CAS No.:127308-98-9
- PACAP 1-27
Catalog No.:BCC5726
CAS No.:127317-03-7
- YLF-466D
Catalog No.:BCC4086
CAS No.:1273323-67-3
- 2-Chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride
Catalog No.:BCC8569
CAS No.:127337-60-4
- Rebaudioside G
Catalog No.:BCN7860
CAS No.:127345-21-5
- Coclauril
Catalog No.:BCN6150
CAS No.:127350-68-9
CaMKII Inhibitor KN-62 Blunts Tumor Response to Hypoxia by Inhibiting HIF-1alpha in Hepatoma Cells.[Pubmed:21165333]
Korean J Physiol Pharmacol. 2010 Oct;14(5):331-6.
In rapidly growing tumors, hypoxia commonly develops due to the imbalance between O(2) consumption and supply. Hypoxia Inducible Factor (HIF)-1alpha is a transcription factor responsible for tumor growth and angiogenesis in the hypoxic microenvironment; thus, its inhibition is regarded as a promising strategy for cancer therapy. Given that CamKII or PARP inhibitors are emerging anticancer agents, we investigated if they have the potential to be developed as new HIF-1alpha-targeting drugs. When treating various cancer cells with the inhibitors, we found that a CamKII inhibitor, KN-62, effectively suppressed HIF-1alpha specifically in hepatoma cells. To examine the effect of KN-62 on HIF-1alpha-driven gene expression, we analyzed the EPO-enhancer reporter activity and mRNA levels of HIF-1alpha downstream genes, such as EPO, LOX and CA9. Both the reporter activity and the mRNA expression were repressed by KN-62. We also found that KN-62 suppressed HIF-1alpha by impairing synthesis of HIF-1alpha protein. Based on these results, we propose that KN-62 is a candidate as a HIF-1alpha-targeting anticancer agent.
N-Arylpiperazine modified analogues of the P2X7 receptor KN-62 antagonist are potent inducers of apoptosis of human primary osteoclasts.[Pubmed:16228288]
J Biomed Sci. 2005 Dec;12(6):1013-20.
The P2X7 nucleotide receptor is an ATP-gated ion channel that plays an important role in bone cell function. Here, we investigated the effects of L: -tyrosine derivatives 1-3 as potent P2X7 antagonists on human primary osteoclasts. We found that the level of expression of P2X7 receptor increased after treatment with the derivatives 1-3, together with the induction of high levels of apoptosis. This effect is associated with activation of caspase-3 and inhibition of expression of IL-6. Interestingly, no pro-apoptotic effect of compounds 1-3 was found on human osteoblasts. Our results suggest that the development of specific P2X7 receptor antagonists may be considered a useful tool to modulate apoptosis of human osteoclasts. Since bone loss due to osteoclast-mediated resorption represents one of the major unsolved problem in osteopenic disorders, the identification of molecules able to induce apoptosis of osteoclasts is of great interest for the development of novel therapeutic strategies.
KN-62 analogues as potent differentiating agents of HL-60 cells.[Pubmed:16963120]
Leuk Res. 2007 May;31(5):683-9.
KN-62, an inhibitor of the calmodulin-dependent protein kinases (CaMKs), enhances the terminal differentiation of retinoic acid sensitive human myeloid leukemia cell lines. In an effort to identify additional CaMK inhibitors that exhibit more potent activity in triggering leukemia cell differentiation, we synthesized 45 analogues of KN-62 and determined their ability to induce HL-60 cell differentiation. Sixteen of these novel analogues exhibited significant differentiation-inducing activity, and one analogue, AS-004, was five times more potent than KN-62 in inhibiting proliferation and inducing differentiation of HL-60 cells. Such KN-62 analogues and/or related compounds may prove useful in treating promyelocytic leukemia.
KN-62, a selective inhibitor of Ca(2+)/calmodulin-dependent protein kinase II, inhibits the lysozyme pre-mRNA splicing in myelomonocytic HD11 cells.[Pubmed:15178421]
Biochem Biophys Res Commun. 2004 Jun 25;319(2):405-9.
The lysozyme primary transcript has been shown to be slowly spliced, particularly in LPS-activated myelomonocytic HD11 cells. In this study, Northern blot analysis shows that the splicing of lysozyme pre-mRNA in LPS-activated cells is significantly delayed by treatment with KN-62, a selective inhibitor of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII), but not with Go 6976 and herbimycin A, inhibitors of Ca(2+)-dependent PKC and PTK, respectively. In vitro kinase assay using autocamtide 2 as specific substrate for CaMKII demonstrates that KN-62, when added to the extract from HD11 cells, inhibits selectively CaMKII activity. Treatment of HD11 cells with cycloheximide, a potent inhibitor of protein synthesis, results in a transient increase in lysozyme pre-mRNA levels, whilst the mature mRNA levels are not increased. Moreover, neither cycloheximide nor KN-62 has any effect on the glyceraldehyde-3-phosphate dehydrogenase pre-mRNA splicing. Together, our results indicate that phosphorylation by CaMKII, and probably new protein synthesis may be required for the lysozyme pre-mRNA processing.
Effects of antagonists at the human recombinant P2X7 receptor.[Pubmed:9720806]
Br J Pharmacol. 1998 Jul;124(6):1314-20.
1. We have used whole-cell patch clamping methods to examine the properties of the recombinant human P2X7 (P2Z) receptor stably expressed in HEK-293 cells. 2. In an extracellular solution with lowered concentrations of divalent cations (zero Mg2+ and 0.5 mM Ca2+), both ATP and the nucleotide analogue, 2'- and 3'-O-(4-benzoylbenzoyl)-adenosine 5'-triphosphate (Bz-ATP) evoked concentration-dependent whole-cell inward currents with maxima of 4658+/-671 and 5385+/-990 pA, respectively, at a holding potential of -90 mV. Current-voltage relationships determined using 100 microM Bz-ATP reversed at -2.7+/-3.1 mV, and did not display significant rectification. 3. Repeated applications of 300 microM Bz-ATP produced inward currents with similar rise-times (approx. 450 ms, 5-95% current development) but with progressively slower 95-5% decay times, with the eighth application of this agonist yielding a decay time of 197% of the first application. 4. Concentration-effect curves to ATP and Bz-ATP produced estimated EC50 values of 780 and 52.4 microM, respectively. Consecutive concentration-effect curves to Bz-ATP produced curves with similar maxima and EC50 values. 5. The non-selective P2 antagonists, pyridoxal-phosphate-6-azophenyl-, 2',4'-disulphonic acid (PPADS) and suramin, both produced concentration-dependent increases in maximal inward currents to Bz-ATP, with IC50 concentrations of approximately 1 microM and 70 microM, respectively. The profile of antagonism produced by PPADS was not that of a competitive antagonist. 6. The isoquinolene derivatives 1-(N,O-bis[5-isoquinolinesulphonyl]-N-methyl-L-tyrosyl)-4-phenylpi perazine (KN-62) and calmidazolium both produced antagonism which was not competitive, with IC50 concentrations of approximately 15 and 100 nM, respectively. HMA (5-(N,N-hexamethylene)- amiloride) was also an effective antagonist at a concentration of 10 microM. The group IIb metal, copper, also displayed antagonist properties at the human P2X7 receptor, reducing the maximum response to Bz-ATP by about 50% at a concentration of 1 microM. 7. These data demonstrate that the human recombinant P2X7 receptor displays functional behaviour which is similar to the recombinant rat P2X7 receptor, but has a distinct pharmacological profile with respect to agonist and antagonist sensitivity.
KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II.[Pubmed:2155222]
J Biol Chem. 1990 Mar 15;265(8):4315-20.
1-[N,O-Bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpipera zine (KN-62), a selective inhibitor of rat brain Ca2+/calmodulin-dependent protein kinase II (Ca2+/CaM kinase II) was synthesized and its inhibitory properties in vitro and in vivo were investigated. KN-62 inhibited phosphorylation of exogenous substrate (chicken gizzard myosin 20-kDa light chain) by Ca2+/CaM kinase II with Ki value of 0.9 microM, but no significant effect up to 100 microM on activities of chicken gizzard myosin light chain kinase, rabbit brain protein kinase C, and bovine heart cAMP-dependent protein kinase type II. KN-62 also inhibited the Ca2+/calmodulin-dependent autophosphorylation of both alpha (50 kDa) and beta (60 kDa) subunits of Ca2+/CaM kinase II dose dependently in the presence or absence of exogenous substrate. Kinetic analysis indicated that this inhibitory effect of KN-62 was competitive with respect to calmodulin. However, KN-62 did not inhibit the activity of autophosphorylated Ca2+/CaM kinase II. Moreover, Ca2+/CaM kinase II bound to a KN-62-coupled Sepharose 4B column, but calmodulin did not. These results suggest that KN-62 affects the interaction between calmodulin and Ca2+/CaM kinase II following inhibition of this kinase activity by directly binding to the calmodulin binding site of the enzyme but does not affect the calmodulin-independent activity of already autophosphorylated (activated) enzyme. We examined the effect of KN-62 on cultured PC12 D pheochromocytoma cells. KN-62 suppressed the A23187 (0.5 microM)-induced autophosphorylation of the 53-kDa subunit of Ca2+/CaM kinase in PC12 D cells, which was immunoprecipitated with anti-rat forebrain Ca2+/CaM kinase II polypeptides antibodies coupled to Sepharose 4B, thereby suggesting that KN-62 could inhibit the Ca2+/CaM kinase II activity in vivo.


