KN-93CaMKII inhibitor,selective and cometitive CAS# 139298-40-1 |
- KN-92 hydrochloride
Catalog No.:BCC1681
CAS No.:1431698-47-3
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- A 438079 hydrochloride
Catalog No.:BCC1317
CAS No.:899431-18-6
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139298-40-1 | SDF | Download SDF |
| PubChem ID | 5312122 | Appearance | Powder |
| Formula | C26H29ClN2O4S | M.Wt | 501.04 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO with gentle warming | ||
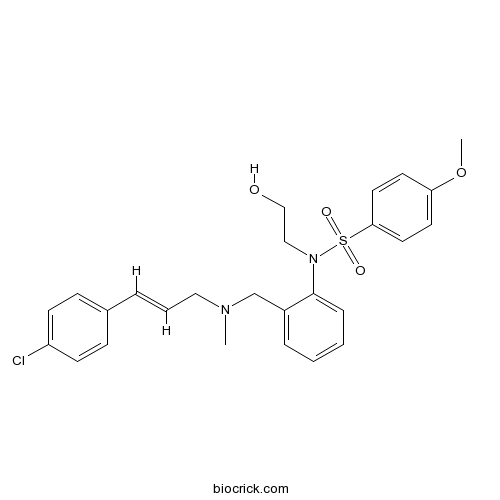
| Chemical Name | N-[2-[[[(E)-3-(4-chlorophenyl)prop-2-enyl]-methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide | ||
| SMILES | CN(CC=CC1=CC=C(C=C1)Cl)CC2=CC=CC=C2N(CCO)S(=O)(=O)C3=CC=C(C=C3)OC | ||
| Standard InChIKey | LLLQTDSSHZREGW-AATRIKPKSA-N | ||
| Standard InChI | InChI=1S/C26H29ClN2O4S/c1-28(17-5-6-21-9-11-23(27)12-10-21)20-22-7-3-4-8-26(22)29(18-19-30)34(31,32)25-15-13-24(33-2)14-16-25/h3-16,30H,17-20H2,1-2H3/b6-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, cell permeable inhibitor of CaM kinase II (IC50 = 0.37 μM). Also a direct extracellular open channel blocker of voltage-gated potassium channels (IC50 = 307 nM for KV1.5) and abolishes IKr in ventricular myocytes (IC50 = 102.6 nM) independently of CaM kinase II inhibition. Water soluble form also available. |

KN-93 Dilution Calculator

KN-93 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9958 mL | 9.9792 mL | 19.9585 mL | 39.917 mL | 49.8962 mL |
| 5 mM | 0.3992 mL | 1.9958 mL | 3.9917 mL | 7.9834 mL | 9.9792 mL |
| 10 mM | 0.1996 mL | 0.9979 mL | 1.9958 mL | 3.9917 mL | 4.9896 mL |
| 50 mM | 0.0399 mL | 0.1996 mL | 0.3992 mL | 0.7983 mL | 0.9979 mL |
| 100 mM | 0.02 mL | 0.0998 mL | 0.1996 mL | 0.3992 mL | 0.499 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KN-93 is a potent and selective inhibitor of CaM kinase II with IC50 value of 0.37μM.[1]
CaM kinase II means Ca2+/calmodulin-dependent protein kinase II which is a serine/threonine-specific protein kinase. It is regulated by the Ca2+/calmodulin complex. CaMKII has 28 different isoforms. The structure governs an autoinhibition which is the Threonine 286 residue. When this site is phosphorylated, it will permanently activate the CaMKII enzyme. The variable and self-associative domains of CaMKII enzyme govern the sensitivity to calcium and calmodulin. CaMKII is related to many signaling pathways. CaMKII is considered to play an important role in learning and memory. CaMKII is also important for reuptake in cardiomyocytes and Ca2+ homeostasis, and CD8 T-cell activation. Misregulation of CaMKII is considered to be related to Alzheimer’s disease, heart arrhythmia , and Angelman syndrome.[2]
KN-93 significantly inhibited CaM kinase activity at 0.5μM in vitro CaM kinase assay. KN-93 potent inhibited CaM kinase II activity In vitro CaM kinase activity with rabbit myocardium with Ki value of 2.58μM. [1] KN-93 inhibited cell growth at 12μM in NIH 3T3 cells and arrested cells in G1 cycle. KN-93 induced cell apoptosis at 24μM in NIH 3T3 cells. [3] KN-93 inhibits expression of Mcl-1which is an anti-apoptotic protein, It also induces p53-independent cell death in PCa cells. KN-93 also induces the generation of ROS and inhibits AR activity then induces cell death.
References:
[1]. Anderson ME, Braun AP, Wu Y, Lu T, Schulman H, Sung RJ: KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther 1998, 287(3):996-1006.
[2]. Yamauchi T: Neuronal Ca2+/calmodulin-dependent protein kinase II--discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol Pharm Bull 2005, 28(8):1342-1354.
[3]. Tombes RM, Grant S, Westin EH, Krystal G: G1 cell cycle arrest and apoptosis are induced in NIH 3T3 cells by KN-93, an inhibitor of CaMK-II (the multifunctional Ca2+/CaM kinase). Cell Growth Differ 1995, 6(9):1063-1070.
- MDL 100907
Catalog No.:BCC7877
CAS No.:139290-65-6
- Zolmitriptan
Catalog No.:BCC5062
CAS No.:139264-17-8
- H2L5186303
Catalog No.:BCC6315
CAS No.:139262-76-3
- Fmoc-Lys-OH.HCl
Catalog No.:BCC3512
CAS No.:139262-23-0
- G-36
Catalog No.:BCC6283
CAS No.:1392487-51-2
- Musellarin C
Catalog No.:BCN7004
CAS No.:1392476-33-3
- Musellarin B
Catalog No.:BCN7192
CAS No.:1392476-32-2
- 3,4-Dihydroxy-2-O-methylanigorufone
Catalog No.:BCN7182
CAS No.:1392307-42-4
- Dodonaflavonol
Catalog No.:BCN6862
CAS No.:1392213-93-2
- 24-Hydroxy-25-ethoxy-3,4-secocycloart-4(28)-en-3-oic acid methyl ester
Catalog No.:BCN7050
CAS No.:1392210-81-9
- Verdinexor (KPT-335)
Catalog No.:BCC5573
CAS No.:1392136-43-4
- Picfeltarraenin X
Catalog No.:BCN2859
CAS No.:1391826-61-1
- Thiostrepton
Catalog No.:BCC7621
CAS No.:1393-48-2
- KPT-330
Catalog No.:BCC4446
CAS No.:1393477-72-9
- TC LPA5 4
Catalog No.:BCC6267
CAS No.:1393814-38-4
- Guan-fu base A
Catalog No.:BCN8491
CAS No.:1394-48-5
- Tiotropium Bromide hydrate
Catalog No.:BCC4585
CAS No.:139404-48-1
- GNE-317
Catalog No.:BCC5655
CAS No.:1394076-92-6
- 8alpha-Hydroxyhirsutinolide
Catalog No.:BCN7111
CAS No.:1394156-45-6
- Boc-Cysteinol(Bzl)
Catalog No.:BCC3043
CAS No.:139428-96-9
- 6-O-apiosyl-5-O-Methylvisammioside
Catalog No.:BCN7858
CAS No.:139446-82-5
- Methyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC9033
CAS No.:139481-28-0
- Ethyl 2-ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylate
Catalog No.:BCC8970
CAS No.:139481-41-7
- Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate
Catalog No.:BCC9032
CAS No.:139481-44-0
Apoptosis induced by NAD depletion is inhibited by KN-93 in a CaMKII-independent manner.[Pubmed:26024774]
Exp Cell Res. 2015 Jul 1;335(1):62-7.
Nicotinamide phosphoribosyltransferase (NAMPT) is a key enzyme that catalyzes the synthesis of nicotinamide mononucleotide from nicotinamide (Nam) in the salvage pathway of mammalian NAD biosynthesis. Several potent NAMPT inhibitors have been identified and used to investigate the role of intracellular NAD and to develop therapeutics. NAD depletion induced by NAMPT inhibitors depolarizes mitochondrial membrane potential and causes apoptosis in a range of cell types. However, the mechanisms behind this depolarization have not been precisely elucidated. We observed that apoptosis of THP-1 cells in response to NAMPT inhibitors was reduced by the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitor KN-93 via an unknown mechanism. The inactive analog of KN-93, KN-92, exhibited the same activity, but the CaMKII-inhibiting cell-permeable autocamtide-2-related inhibitory peptide II did not, indicating that the inhibition of THP-1 cell apoptosis was not dependent on CaMKII. In evaluating the mechanism of action, we confirmed that KN-93 did not inhibit decreases in NAD levels but did inhibit decreases in mitochondrial membrane potential, indicating that KN-93 exerts inhibition upstream of the mitochondrial pathway of apoptosis. Further, qPCR analysis of the Bcl-2 family of proteins showed that Bim is efficiently expressed following NAMPT inhibition and that KN-92 did not inhibit this expression. The L-type Ca(2+) channel blockers verapamil and nimodipine partially inhibited apoptosis, indicating that part of this effect is dependent on Ca(2+) channel inhibition, as both KN-93 and KN-92 are reported to inhibit L-type Ca(2+) channels. On the other hand, KN-93 and KN-92 did not markedly inhibit apoptosis induced by anti-cancer agents such as etoposide, actinomycin D, ABT-737, or TW-37, indicating that the mechanism of inhibition is specific to apoptosis induced by NAD depletion. These results demonstrate that NAD depletion induces a specific type of apoptosis that is effectively inhibited by the KN-93 series of compounds.
KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3(+) regulatory T cells in MRL/lpr mice.[Pubmed:24829059]
Autoimmunity. 2014 Nov;47(7):445-50.
OBJECTIVE: Foxp3(+) regulatory T cells (Treg) are pivotal for the maintenance of peripheral tolerance and prevent development of autoimmune diseases. We have reported that calcium/calmodulin-dependent protein kinase IV (CaMK4) deficient MRL/lpr mice display less disease activity by promoting IL-2 production and increasing the activity of Treg cells. To further define the mechanism of CaMK4 on Treg cells in systemic lupus erythematosus (SLE), we used the Foxp3-GFP reporter mice and treated them with KN-93, an inhibitor of CaMK4. METHODS: We generated MRL/lpr Foxp3-GFP mice to record Treg cells; stimulated naive CD4(+) T cells from MRL/lpr Foxp3-GFP mice under Treg polarizing conditions in the absence or presence of KN-93; evaluated the number of GFP positive cells in lymphoid organs and examined skin and kidney pathology at 16 weeks of age. We also examined the infiltration of cells and recruitment of Treg cells in the kidney. RESULTS: We show that culture of MRL/lpr Foxp3-GFP T cells in the presence of KN-93 promotes Treg differentiation in a dose-dependent manner. Treatment of MRL/lpr Foxp3-GFP mice with KN-93 results in a significant induction of Treg cells in the spleen, peripheral lymph nodes and peripheral blood and this is accompanied by decreased skin and kidney damage. Notably, KN-93 clearly diminishes the accumulation of inflammatory cells along with reciprocally increased Treg cells in target organ. CONCLUSION: Our results indicate that KN-93 treatment enhances the generation of Treg cells in vitro and in vivo highlighting its potential therapeutic use for the treatment of human autoimmune diseases.
KN-93, A CaMKII inhibitor, suppresses ventricular arrhythmia induced by LQT2 without decreasing TDR.[Pubmed:24142712]
J Huazhong Univ Sci Technolog Med Sci. 2013 Oct;33(5):636-639.
Abnormal enhanced transmural dispersion of repolarization (TDR) plays an important role in the maintaining of the severe ventricular arrhythmias such as torsades de pointes (TDP) which can be induced in long-QT (LQT) syndrome. Taking advantage of an in vitro rabbit model of LQT2, we detected the effects of KN-93, a CaM-dependent kinase (CaMK) II inhibitor on repolarization heterogeneity of ventricular myocardium. Using the monophasic action potential recording technique, the action potentials of epicardium and endocardium were recorded in rabbit cardiac wedge infused with hypokalemic, hypomagnesaemic Tyrode's solution. At a basic length (BCL) of 2000 ms, LQT2 model was successfully mimicked with the perfusion of 0.5 mumol/L E-4031, QT intervals and the interval from the peak of T wave to the end of T wave (Tp-e) were prolonged, and Tp-e/QT increased. Besides, TDR was increased and the occurrence rate of arrhythmias like EAD, R-on-T extrasystole, and TDP increased under the above condition. Pretreatment with KN-93 (0.5 mumol/L) could inhibit EAD, R-on-T extrasystole, and TDP induced by E-4031 without affecting QT interval, Tp-e, and Tp-e/QT. This study demonstrated KN-93, a CaMKII inhibitor, can inhibit EADs which are the triggers of TDP, resulting in the suppression of TDP induced by LQT2 without affecting TDR.
KN-93 inhibits IKr in mammalian cardiomyocytes.[Pubmed:26463508]
J Mol Cell Cardiol. 2015 Dec;89(Pt B):173-6.
Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibitor KN-93 is widely used in multiple fields of cardiac research especially for studying the mechanisms of cardiomyopathy and cardiac arrhythmias. Whereas KN-93 is a potent inhibitor of CaMKII, several off-target effects have also been found in expression cell systems and smooth muscle cells, but there is no information on the KN93 side effects in mammalian ventricular myocytes. In this study we explore the effect of KN-93 on the rapid component of delayed rectifier potassium current (IKr) in the ventricular myocytes from rabbit and guinea pig hearts. Our data indicate that KN-93 exerts direct inhibitory effect on IKr that is not mediated via CaMKII. This off-target effect of KN93 should be taken into account when interpreting the data from using KN93 to investigate the role of CaMKII in cardiac function.
KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N -methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels.[Pubmed:16368898]
J Pharmacol Exp Ther. 2006 Apr;317(1):292-9.
The effect of Ca(2+)/calmodulin-dependent protein kinase II (CaMK II) on voltage-gated ion channels is widely studied through the use of specific CaMK II blockers such as 2-[N-(2-hydroxyethyl)]-N-(4methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-m ethylbenzylamine (KN-93). The present study demonstrates that KN-93 is a direct extracellular blocker of a wide range of cloned Kv channels from a number of different subfamilies. In all channels tested, the effect of 1 microM KN-93 was independent of CaMK II because 1 microM2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylam ine, phosphate (KN-92), an inactive analog of KN-93, caused similar inhibition of currents. In addition, dialysis of cells with 10 microM CaMK II inhibitory peptide fragment 281-301 (CIP) had no effect on current kinetics and did not prevent the inhibitory effect of KN-93. The IC(50) for block of the Kv1.5 channel (used as an example to determine the nature of KN-93 block) was 307 +/- 12 nM. KN-93 blocked open channels with little voltage dependence that did not alter the V(1/2) of channel activation. Removal of P/C-type inactivation by mutation of arginine 487 to valine in the outer pore region of Kv1.5 (R487V) greatly reduced KN-93 block, whereas enhancement of inactivation induced by mutation of threonine 462 to cysteine (T462C) increased the potency of KN-93 by 4-fold. This suggested that KN-93 acted through promotion and stabilization of C-type inactivation. Importantly, KN-93 was ineffective as a blocker when applied intracellularly, suggesting that CaMK II-independent effects of KN-93 on Kv channels can be circumvented by intracellular application of KN-93.
Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells.[Pubmed:10075693]
J Biol Chem. 1999 Mar 19;274(12):7958-68.
The human tyrosine phosphatase (p54(cdc25-c)) is activated by phosphorylation at mitosis entry. The phosphorylated p54(cdc25-c) in turn activates the p34-cyclin B protein kinase and triggers mitosis. Although the active p34-cyclin B protein kinase can itself phosphorylate and activate p54(cdc25-c), we have investigated the possibility that other kinases may initially trigger the phosphorylation and activation of p54(cdc25-c). We have examined the effects of the calcium/calmodulin-dependent protein kinase (CaM kinase II) on p54(cdc25-c). Our in vitro experiments show that CaM kinase II can phosphorylate p54(cdc25-c) and increase its phosphatase activity by 2.5-3-fold. Treatment of a synchronous population of HeLa cells with KN-93 (a water-soluble inhibitor of CaM kinase II) or the microinjection of AC3-I (a specific peptide inhibitor of CaM kinase II) results in a cell cycle block in G2 phase. In the KN-93-arrested cells, p54(cdc25-c) is not phosphorylated, p34(cdc2) remains tyrosine phosphorylated, and there is no increase in histone H1 kinase activity. Our data suggest that a calcium-calmodulin-dependent step may be involved in the initial activation of p54(cdc25-c).
The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells.[Pubmed:1662507]
Biochem Biophys Res Commun. 1991 Dec 31;181(3):968-75.
We reported that one of the isoquinolinesulfonamide derivatives, KN-62, is a potent and specific inhibitor of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Tokumitsu, H., Chijiwa, T., Hagiwara, M., Mizutani, A., Terasawa, M. and Hidaka, H. (1990) J. Biol. Chem. 265, 4315-4320). We have now investigated the inhibitory property of a newly synthesized methoxybenzenesulfonamide, KN-93, on CaMKII activity in situ and in vitro. KN-93 elicited potent inhibitory effects on CaMKII phosphorylating activity with an inhibition constant of 0.37 microM but this compound had no significant effects on the catalytic activity of cAMP-dependent protein kinase, Ca2+/phospholipid dependent protein kinase, myosin light chain kinase and Ca(2+)-phosphodiesterase. KN-93 also inhibited the autophosphorylation of both the alpha- and beta-subunits of CaMKII. Kinetic analysis indicated that KN-93 inhibits CaMKII, in a competitive fashion against calmodulin. To evaluate the regulatory role of CaMKII on catecholamine metabolism, we examined the effect of KN-93 on dopamine (DA) levels in PC12h cells. The DA levels decreased in the presence of KN-93. Further, the tyrosine hydroxylase (TH) phosphorylation induced by KCl or acetylcholine was significantly suppressed by KN-93 in PC12h cells while events induced by forskolin or 8-Br-cAMP were not affected. These results suggest that KN-93 inhibits DA formation by modulating the reaction rate of TH to reduce the Ca(2+)-mediated phosphorylation levels of the TH molecule.


