H2L5186303Potent and selective LPA2 receptor antagonist CAS# 139262-76-3 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139262-76-3 | SDF | Download SDF |
| PubChem ID | 90488981 | Appearance | Powder |
| Formula | C26H20N2O8 | M.Wt | 488.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
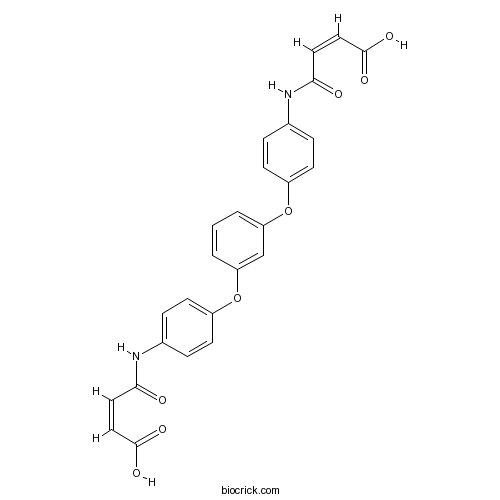
| Chemical Name | (Z)-4-[4-[3-[4-[[(Z)-3-carboxyprop-2-enoyl]amino]phenoxy]phenoxy]anilino]-4-oxobut-2-enoic acid | ||
| SMILES | C1=CC(=CC(=C1)OC2=CC=C(C=C2)NC(=O)C=CC(=O)O)OC3=CC=C(C=C3)NC(=O)C=CC(=O)O | ||
| Standard InChIKey | HZFPOTBCYPWQSH-DZDAAMPGSA-N | ||
| Standard InChI | InChI=1S/C26H20N2O8/c29-23(12-14-25(31)32)27-17-4-8-19(9-5-17)35-21-2-1-3-22(16-21)36-20-10-6-18(7-11-20)28-24(30)13-15-26(33)34/h1-16H,(H,27,29)(H,28,30)(H,31,32)(H,33,34)/b14-12-,15-13- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective lysophosphatidic acid 2 (LPA2) receptor antagonist (IC50 values are 8.9, 1230 and 27354 nM for LPA2, LPA3 and LPA1 receptors respectively, in a LPA-elicited calcium mobilization assay). |

H2L5186303 Dilution Calculator

H2L5186303 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0473 mL | 10.2365 mL | 20.4729 mL | 40.9458 mL | 51.1823 mL |
| 5 mM | 0.4095 mL | 2.0473 mL | 4.0946 mL | 8.1892 mL | 10.2365 mL |
| 10 mM | 0.2047 mL | 1.0236 mL | 2.0473 mL | 4.0946 mL | 5.1182 mL |
| 50 mM | 0.0409 mL | 0.2047 mL | 0.4095 mL | 0.8189 mL | 1.0236 mL |
| 100 mM | 0.0205 mL | 0.1024 mL | 0.2047 mL | 0.4095 mL | 0.5118 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Lys-OH.HCl
Catalog No.:BCC3512
CAS No.:139262-23-0
- G-36
Catalog No.:BCC6283
CAS No.:1392487-51-2
- Musellarin C
Catalog No.:BCN7004
CAS No.:1392476-33-3
- Musellarin B
Catalog No.:BCN7192
CAS No.:1392476-32-2
- 3,4-Dihydroxy-2-O-methylanigorufone
Catalog No.:BCN7182
CAS No.:1392307-42-4
- Dodonaflavonol
Catalog No.:BCN6862
CAS No.:1392213-93-2
- 24-Hydroxy-25-ethoxy-3,4-secocycloart-4(28)-en-3-oic acid methyl ester
Catalog No.:BCN7050
CAS No.:1392210-81-9
- Verdinexor (KPT-335)
Catalog No.:BCC5573
CAS No.:1392136-43-4
- Picfeltarraenin X
Catalog No.:BCN2859
CAS No.:1391826-61-1
- ZM 241385
Catalog No.:BCC6902
CAS No.:139180-30-6
- CEP-37440
Catalog No.:BCC5145
CAS No.:1391712-60-9
- H-Tle-OH.HCl
Catalog No.:BCC2660
CAS No.:139163-43-2
- Zolmitriptan
Catalog No.:BCC5062
CAS No.:139264-17-8
- MDL 100907
Catalog No.:BCC7877
CAS No.:139290-65-6
- KN-93
Catalog No.:BCC1683
CAS No.:139298-40-1
- Thiostrepton
Catalog No.:BCC7621
CAS No.:1393-48-2
- KPT-330
Catalog No.:BCC4446
CAS No.:1393477-72-9
- TC LPA5 4
Catalog No.:BCC6267
CAS No.:1393814-38-4
- Guan-fu base A
Catalog No.:BCN8491
CAS No.:1394-48-5
- Tiotropium Bromide hydrate
Catalog No.:BCC4585
CAS No.:139404-48-1
- GNE-317
Catalog No.:BCC5655
CAS No.:1394076-92-6
- 8alpha-Hydroxyhirsutinolide
Catalog No.:BCN7111
CAS No.:1394156-45-6
- Boc-Cysteinol(Bzl)
Catalog No.:BCC3043
CAS No.:139428-96-9
- 6-O-apiosyl-5-O-Methylvisammioside
Catalog No.:BCN7858
CAS No.:139446-82-5
Lysophosphatidic acid acts on LPA1 receptor to increase H2 O2 during flow-induced dilation in human adipose arterioles.[Pubmed:30153326]
Br J Pharmacol. 2018 Nov;175(22):4266-4280.
BACKGROUND AND PURPOSE: NO produces arteriolar flow-induced dilation (FID) in healthy subjects but is replaced by mitochondria-derived hydrogen peroxide (mtH2 O2 ) in patients with coronary artery disease (CAD). Lysophosphatidic acid (LPA) is elevated in patients with risk factors for CAD, but its functional effect in arterioles is unknown. We tested whether elevated LPA changes the mediator of FID from NO to mtH2 O2 in human visceral and subcutaneous adipose arterioles. EXPERIMENTAL APPROACH: Arterioles were cannulated on glass micropipettes and pressurized to 60 mmHg. We recorded lumen diameter after graded increases in flow in the presence of either NOS inhibition (L-NAME) or H2 O2 scavenging (Peg-Cat) +/- LPA (10 muM, 30 min), +/-LPA1 /LPA3 receptor antagonist (Ki16425) or LPA2 receptor antagonist (H2L5186303). We analysed LPA receptor RNA and protein levels in human arterioles and human cultured endothelial cells. KEY RESULTS: FID was inhibited by L-NAME but not Peg-Cat in untreated vessels. In vessels treated with LPA, FID was of similar magnitude but inhibited by Peg-Cat while L-NAME had no effect. Rotenone attenuated FID in vessels treated with LPA indicating mitochondria as a source of ROS. RNA transcripts from LPA1 and LPA2 but not LPA3 receptors were detected in arterioles. LPA1 but not LPA3 receptor protein was detected by Western blot. Pretreatment of vessels with an LPA1 /LPA3 , but not LPA2 , receptor antagonist prior to LPA preserved NO-mediated dilation. CONCLUSIONS AND IMPLICATIONS: These findings suggest an LPA1 receptor-dependent pathway by which LPA increases arteriolar release of mtH2 O2 as a mediator of FMD.
LPA1 is a key mediator of intracellular signalling and neuroprotection triggered by tetracyclic antidepressants in hippocampal neurons.[Pubmed:28815598]
J Neurochem. 2017 Oct;143(2):183-197.
Both lysophosphatidic acid (LPA) and antidepressants have been shown to affect neuronal survival and differentiation, but whether LPA signalling participates in the action of antidepressants is still unknown. In this study, we examined the role of LPA receptors in the regulation of extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activity and neuronal survival by the tetracyclic antidepressants, mianserin and mirtazapine in hippocampal neurons. In HT22 immortalized hippocampal cells, antidepressants and LPA induced a time- and concentration-dependent stimulation of ERK1/2 phosphorylation. This response was inhibited by either LPA1 and LPA1/3 selective antagonists or siRNA-induced LPA1 down-regulation, and enhanced by LPA1 over-expression. Conversely, the selective LPA2 antagonist H2L5186303 had no effect. Antidepressants induced cyclic AMP response element binding protein phosphorylation and this response was prevented by LPA1 blockade. ERK1/2 stimulation involved pertussis toxin-sensitive G proteins, Src tyrosine kinases and fibroblast growth factor receptor (FGF-R) activity. Tyrosine phosphorylation of FGF-R was enhanced by antidepressants through LPA1 . Serum withdrawal induced apoptotic death, as indicated by increased annexin V staining, caspase activation and cleavage of poly-ADP-ribose polymerase. Antidepressants inhibited the apoptotic cascade and this protective effect was curtailed by blockade of either LPA1 , ERK1/2 or FGF-R activity. Moreover, in primary mouse hippocampal neurons, mianserin acting through LPA1 increased phospho-ERK1/2 and protected from apoptosis induced by removal of growth supplement. These data indicate that in neurons endogenously expressed LPA1 receptors mediate intracellular signalling and neuroprotection by tetracyclic antidepressants.
Structure-based drug design identifies novel LPA3 antagonists.[Pubmed:19800804]
Bioorg Med Chem. 2009 Nov 1;17(21):7457-64.
Compound 5 ([5-(3-nitrophenoxy)-1,3-dioxo-1,3-dihydro-2-isoindol-2-yl]acetic acid) was identified as a weak selective LPA(3) antagonist (IC(50)=4504 nM) in a virtual screening effort to optimize a dual LPA(2 and 3) antagonist. Structure-based drug design techniques were used to prioritize similarity search matches of compound 5. This strategy rapidly identified 10 novel antagonists. The two most efficacious compounds identified inhibit activation of the LPA(3) receptor by 200 nM LPA with IC(50) values of 752 nM and 2992 nM. These compounds additionally define changes to our previously reported pharmacophore that will improve its ability to identify more potent and selective LPA(3) receptor antagonists. The results of the combined computational and experimental screening are reported.
Identification of non-lipid LPA3 antagonists by virtual screening.[Pubmed:18467108]
Bioorg Med Chem. 2008 Jun 1;16(11):6207-17.
In the present study, we utilized virtual screening to identify LPA(3) antagonists. We have developed a three-point structure-based pharmacophore model based on known LPA(3) antagonists. This model was used to mine the NCI database. Docking, pharmacophore development, and database mining produced new, non-lipid leads. Experimental testing of seven computationally selected pharmacophore hits produced one potentiator and three antagonists, one of which displays both LPA(3) selectivity and nanomolar potency. Similarity searching in the ChemBridge database using the most promising lead as the search target produced four additional LPA(3) antagonists and a potent dual LPA(1&2) antagonist.


