Hydroxyfasudil hydrochlorideRho-kinase inhibitor and vasodilator CAS# 155558-32-0 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155558-32-0 | SDF | Download SDF |
| PubChem ID | 11371328 | Appearance | Powder |
| Formula | C14H18ClN3O3S | M.Wt | 343.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Hydroxyfasudil | ||
| Solubility | DMSO : 30 mg/mL (87.25 mM; Need ultrasonic) | ||
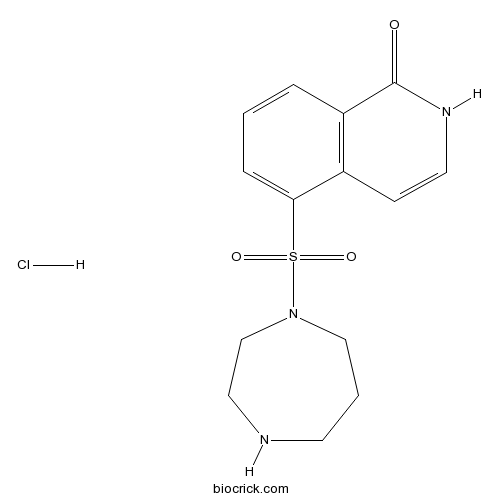
| Chemical Name | 5-(1,4-diazepan-1-ylsulfonyl)-2H-isoquinolin-1-one;hydrochloride | ||
| SMILES | C1CNCCN(C1)S(=O)(=O)C2=CC=CC3=C2C=CNC3=O.Cl | ||
| Standard InChIKey | XWWFOUVDVJGNNG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H17N3O3S.ClH/c18-14-12-3-1-4-13(11(12)5-7-16-14)21(19,20)17-9-2-6-15-8-10-17;/h1,3-5,7,15H,2,6,8-10H2,(H,16,18);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable active metabolite of Fasudil. Produces ATP-competitive and reversible inhibition of Rho-kinase and is ~ 100-fold selective over a range of other protein kinases. Inhibits neutrophil migration and produces potent vasodilatory effects in vivo. |

Hydroxyfasudil hydrochloride Dilution Calculator

Hydroxyfasudil hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9084 mL | 14.5421 mL | 29.0841 mL | 58.1683 mL | 72.7104 mL |
| 5 mM | 0.5817 mL | 2.9084 mL | 5.8168 mL | 11.6337 mL | 14.5421 mL |
| 10 mM | 0.2908 mL | 1.4542 mL | 2.9084 mL | 5.8168 mL | 7.271 mL |
| 50 mM | 0.0582 mL | 0.2908 mL | 0.5817 mL | 1.1634 mL | 1.4542 mL |
| 100 mM | 0.0291 mL | 0.1454 mL | 0.2908 mL | 0.5817 mL | 0.7271 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 0.12 uM (ROCK1); 0.17 uM (ROCK2) [1] Hydroxyfasudil, metabolite of Fasudil, is a potent Rho-kinase inhibitor and vasodilator. in vitro: Fasudil (1-10 μM) and hydroxyfasudil (0.3-10 μM) significantly prevented endothelin-induced cardiomyocyte hypertrophy [2]. Hydroxyfasudil significantly attenuated serotonin (IC)-induced vasoconstriction of SA (-7 +/- 1% vs. 2 +/- 1%, p < 0.01). Coronary I/R significantly impaired coronary vasodilation to acetylcholine after I/R (SA, p < 0.05; and A, p < 0.01 vs. before I/R) and L-NMMA further reduced the vasodilation, whereas hydroxyfasudil completely preserved the responses. in vivo: Treatment with hydroxyfasudil significantly improved bladder intercontraction intervals. Rats treated with hydroxyfasudil also showed a significant reduction of histopathological features associated with cystitis [3]. Twelve-week-old male SHRs were treated with hydroxyfasudil (3 or 10 mg/kg, i.p.) once a day for 6 weeks. Treatment with hydroxyfasudil significantly improved the decreased penile cGMP concentrations, the increased Rho kinase activities, the increased norepinephrine-induced contractions, and the decreased acetylcholine-induced relaxation in a dose-dependent manner [4]. Toxicity: The proportion of patients with good clinical outcome was 74.5% (41/55) in the fasudil group and 61.7% (37/60) in the nimodipine group. There were no serious adverse events reported in the fasudil group [5]. Clinical trial: N/A
- 3a-Epiburchellin
Catalog No.:BCN7015
CAS No.:155551-61-4
- Alisol G
Catalog No.:BCN3461
CAS No.:155521-46-3
- Alisol F
Catalog No.:BCN3360
CAS No.:155521-45-2
- DIPPA hydrochloride
Catalog No.:BCC6799
CAS No.:155512-52-0
- 1,9-Caryolanediol 9-acetate
Catalog No.:BCN1698
CAS No.:155488-34-9
- 3,6-Caryolanediol
Catalog No.:BCN1697
CAS No.:155485-76-0
- JWH 015
Catalog No.:BCC5744
CAS No.:155471-08-2
- 4,5-Dihydroblumenol A
Catalog No.:BCN1696
CAS No.:155418-97-6
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- VUF 10166
Catalog No.:BCC5060
CAS No.:155584-74-0
- Dehydroaglaiastatin
Catalog No.:BCN1699
CAS No.:155595-93-0
- PG-9 maleate
Catalog No.:BCC6779
CAS No.:155649-00-6
- Notoginsenoside Ft1
Catalog No.:BCN6434
CAS No.:155683-00-4
- Simonsinol
Catalog No.:BCN1700
CAS No.:155709-40-3
- Isomagnolone
Catalog No.:BCN1701
CAS No.:155709-41-4
- Mecarbinate
Catalog No.:BCC4919
CAS No.:15574-49-9
- Pizotifen
Catalog No.:BCC4215
CAS No.:15574-96-6
- Peujaponiside
Catalog No.:BCN8261
CAS No.:155740-16-2
- Hierochin D
Catalog No.:BCN1702
CAS No.:155759-02-7
- 4-IBP
Catalog No.:BCC6777
CAS No.:155798-08-6
- 3-beta-O-(cis-p-Coumaroyl)corosolic acid
Catalog No.:BCN1553
CAS No.:155800-17-2
Chronologic changes of fasudil hydrochloride and hydroxyfasudil in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage.[Pubmed:17903999]
J Stroke Cerebrovasc Dis. 2005 Mar-Apr;14(2):47-9.
Fasudil hydrochloride (FH) has been developed as an antivasospasm agent. Its dynamics in cerebrospinal fluid (CSF) and the vasodilating action of hydroxyfasudil (M3) have been obscure, although FH dilates spastic ateries from the inside of the vessel wall. The present study investigated concentrations of FH and M3 in serum and CSF. Dynamic studies of FH and M3 in the CSF of 10 patients with subarachnoid hemorrhage were conducted. FH (30 mg) was injected intravenously for 30 minutes, 3 times a day. Intra-arterial injection using a microcatheter from intracranial portions of the internal carotid artery was added to 3 patients with severe vasospasm. M3 remained in the serum longer than FH. Approximately 20% of the FH and M3 was transferred to CSF and remained there for a long time. The intra-arterial injections significantly increased M3 levels in CSF. These basic data may be helpful in developing future treatments.
Hydroxyfasudil, an active metabolite of fasudil hydrochloride, relaxes the rabbit basilar artery by disinhibition of myosin light chain phosphatase.[Pubmed:11435800]
J Cereb Blood Flow Metab. 2001 Jul;21(7):876-85.
Fasudil hydrochloride (AT877, hexahydro-1-(5-isoquinolinesulfonyl)-1H-1,4-diazepine hydrochloride, identical to HA1077) inhibits cerebral vasospasm after subarachnoid hemorrhage in experimental animals and humans. In the current study, the vasorelaxing mechanism of hydroxyfasudil, a hydroxylated metabolite of fasudil hydrochloride, was determined in the rabbit basilar artery. The effects of hydroxyfasudil on tension, intracellular Ca2+ concentration ([Ca2+]i), and phosphorylation of the myosin light chain were examined using the isolated and intact or permeabilized rabbit basilar artery without endothelium in vitro. In the intact rabbit basilar artery, hydroxyfasudil elicited a concentration-dependent relaxation of the artery precontracted with 1 nmol/L endothelin-1 (ET-1) plus 20 mmol/L KCl without any significant decrease in [Ca2+]i as determined by fura-2 microfluorometry (IC50: 5.1 +/- 4.6 micromol/L). The relaxation induced by hydroxyfasudil was accompanied with dephosphorylation of the myosin light chain. In the permeabilized preparation, hydroxyfasudil inhibited the contraction induced by ET-1, guanosine 5'-O-(3-thiotriphosphate), or the catalytic subunit of rho-associated kinase, but it did not inhibit Ca2+-induced contraction under the condition of inhibited myosin light chain phosphatase. Hydroxyfasudil showed a greater relaxant effect under decreased adenosine triphosphate (ATP) levels. The present study indicated that hydroxyfasudil relaxes the rabbit basilar artery mainly by disinhibiting myosin light chain phosphatase through the inhibition of rho-associated kinase and that this effect depends on the intracellular ATP concentration.
Antianginal effects of hydroxyfasudil, a Rho-kinase inhibitor, in a canine model of effort angina.[Pubmed:11739249]
Br J Pharmacol. 2001 Dec;134(8):1724-30.
1. The effects of Rho-kinase inhibitor, fasudil, and of a more specific Rho-kinase inhibitor, hydroxyfasudil, on pacing-induced myocardial ischaemia were determined in anaesthetized open-chest dogs. 2. The dogs were subjected to left anterior descending coronary artery (LAD) stenosis producing a sufficient ischaemia as measured by ST-segment depression on electrocardiograms only when the hearts were paced 60 beats min(-1) above the baseline. After a recovery (nonpacing) period, drugs or saline were infused intravenously over 30 min. The animals were again subjected to 5 min of pacing 25 min after the initiation of the treatment. 3. Hydroxyfasudil (0.1 and 0.3 mg kg(-1)) and fasudil (0.3 mg kg(-1)) suppressed the ST-segment depression. Hydroxyfasudil and fasudil also increased the regional blood flow of the LAD perfused endomyocardium region in the canine model of effort angina. 4. To determine the flow profile for hydroxyfasudil in dogs, blood flow in three vascular beds was measured. Hydroxyfasudil (0.3 mg kg(-1) for 30 min) significantly increased coronary blood flow and vertebral blood flow, without significantly changing the femoral blood flow. 5. Hydroxyfasudil had no inotropic or chronotropic effect on the isolated hearts of guinea-pigs. Hydroxyfasudil (2 mg kg(-1) for 20 min) did not affect the PR or QTc interval in anaesthetized dogs. 6. Inhibition of Rho-kinase appears to protect myocardium subjected to pacing-induced ischaemia through the increase in the regional myocardial blood flow. Hydroxyfasudil may be categorized as a novel type of anti-anginal drug, without any inotropic or chronotropic effects.
Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm.[Pubmed:10615430]
Cardiovasc Res. 1999 Sep;43(4):1029-39.
OBJECTIVE: We recently demonstrated in our swine model of coronary artery spasm that enhanced myosin light chain (MLC) phosphorylations (both MLC mono- and diphosphorylations) play a central role in the pathogenesis of the spasm. However, the molecular mechanism for and the phosphorylation sites for the enhanced MLC phosphorylations were unknown. In the present study, we addressed these points using hydroxyfasudil, a novel inhibitor of protein kinases, which we found preferentially inhibits Rho-kinase. METHODS: The specificity of the inhibitory effects of hydroxyfasudil on Rho-kinase, MLCK, MRCK beta and PKC were examined by kinase assay in vitro. The left porcine coronary artery was chronically treated with interleukin-1 beta (IL-1 beta, 2.5 micrograms). Two weeks after the operation, coronary artery vasomotion was examined both in vivo and in vitro. MLC phosphorylations were examined by Western blot analysis and the sites for the phosphorylations by anti-phosphorylated MLC antibodies that identified the monophosphorylation site as Ser19 and diphophorylation sites as Ser19/Thr18 of MLC. RESULTS: Inhibitory effects of hydroxyfasudil was at least 100 times more potent for Rho-kinase as compared with other protein kinases tested. Intracoronary serotonin (10 micrograms/kg) caused coronary hyperconstriction at the IL-1 beta-treated site in vivo, which was dose-dependently inhibited by hydroxyfasudil (p < 0.01). The coronary segment taken from the spastic site also showed hypercontractions to serotonin in vitro, which were again dose-dependently inhibited by hydroxyfasudil (p < 0.01). Western blot analysis showed that MLC monophosphorylation was significantly greater in the spastic segment than in the control segment, while MLC diphosphorylation was noted only at the spastic segment (p < 0.01). The sites for the mono- and diphosphorylated MLC were identified as the monophosphorylated site Ser19 and diphosphorylated sites Ser19/Thr18 of MLC, respectively. Both types of MLC phosphorylations at the spastic segment were markedly inhibited by hydroxyfasudil (p < 0.01). CONCLUSION: These results indicate that hydroxyfasudil-sensitive Rho-kinase-mediated pathway appears to mediate the enhanced MLC phosphorylations (on Ser19 and Ser19/Thr18 residues) and plays a central role in the pathogenesis of coronary artery spasm.
Inhibition by the protein kinase inhibitor HA1077 of the activation of NADPH oxidase in human neutrophils.[Pubmed:8240400]
Biochem Pharmacol. 1993 Oct 19;46(8):1487-90.
The effect of an inhibitor of protein kinase, HA1077 [1-(5-isoquinolinesulfonyl)-homopiperazine HCl], and its hydroxylated metabolite, HA1100, on the activation of NADPH oxidase in human neutrophils were studied. Cells were preincubated with each drug for 10 min and then activated by treatment with phorbol myristate acetate (PMA) or formylmethionyl leucyl phenylalanine (FMLP). After activation, the rate of superoxide dismutase-inhibitable reduction of cytochrome c was estimated. HA1077 and HA1100 inhibited the PMA-induced production of O2- by neutrophil NADPH oxidase in a concentration-dependent manner (IC50 = 15 and 24 microM, respectively). The sensitivity of the FMLP-induced production of O2- to these drugs was similar. The production of O2- in 1,25-dihydroxyvitamin D3-treated HL-60 cells, which differentiated to macrophage-like cells, was also inhibited by the drugs. The extent of inhibition by HA1077 was almost the same as that by a calmodulin inhibitor (W-7) and by inhibitors of protein kinase (H-7 and H-8). In a cell-free lysate of neutrophils, the NADPH-dependent production of O2- can be induced by sodium dodecyl sulfate (SDS). HA1077 at 100 microM had only a weak inhibitory effect on the cell-free, SDS-induced production of O2-, an indication that HA1077 inhibits the activation of NADPH oxidase, not the actual activity. The effects of H-7 and H-8 were similar to that of HA1077, whereas W-7 inhibited the production of O2- by the cell-free extract of HL-60 cells. This action of HA1077 could explain, in part, its ability to protect neuronal cells from death after ischemia.


