4,5-Dihydroblumenol ACAS# 155418-97-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155418-97-6 | SDF | Download SDF |
| PubChem ID | 21630916 | Appearance | Powder |
| Formula | C13H22O3 | M.Wt | 226.3 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
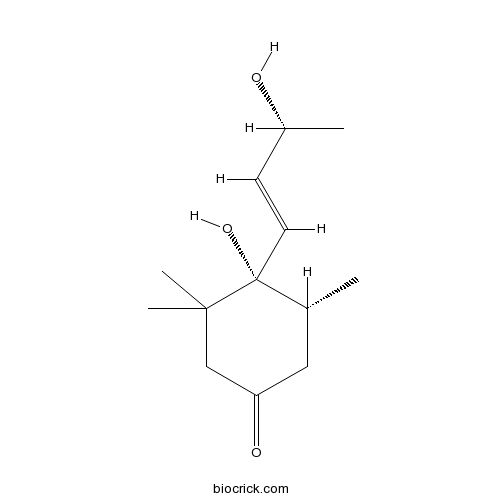
| Chemical Name | (4S,5R)-4-hydroxy-4-[(E,3R)-3-hydroxybut-1-enyl]-3,3,5-trimethylcyclohexan-1-one | ||
| SMILES | CC1CC(=O)CC(C1(C=CC(C)O)O)(C)C | ||
| Standard InChIKey | IHDJYDVWNNFPHR-CHESLIBASA-N | ||
| Standard InChI | InChI=1S/C13H22O3/c1-9-7-11(15)8-12(3,4)13(9,16)6-5-10(2)14/h5-6,9-10,14,16H,7-8H2,1-4H3/b6-5+/t9-,10-,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 4, 5-Dihydroblumenol shows significant inhibition against HepG2 cells transected with cloned hepatitis B virus DNA. |
| Targets | HBV |

4,5-Dihydroblumenol A Dilution Calculator

4,5-Dihydroblumenol A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4189 mL | 22.0946 mL | 44.1891 mL | 88.3783 mL | 110.4728 mL |
| 5 mM | 0.8838 mL | 4.4189 mL | 8.8378 mL | 17.6757 mL | 22.0946 mL |
| 10 mM | 0.4419 mL | 2.2095 mL | 4.4189 mL | 8.8378 mL | 11.0473 mL |
| 50 mM | 0.0884 mL | 0.4419 mL | 0.8838 mL | 1.7676 mL | 2.2095 mL |
| 100 mM | 0.0442 mL | 0.2209 mL | 0.4419 mL | 0.8838 mL | 1.1047 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- Peraksine
Catalog No.:BCN1694
CAS No.:15527-80-7
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- JWH 015
Catalog No.:BCC5744
CAS No.:155471-08-2
- 3,6-Caryolanediol
Catalog No.:BCN1697
CAS No.:155485-76-0
- 1,9-Caryolanediol 9-acetate
Catalog No.:BCN1698
CAS No.:155488-34-9
- DIPPA hydrochloride
Catalog No.:BCC6799
CAS No.:155512-52-0
- Alisol F
Catalog No.:BCN3360
CAS No.:155521-45-2
- Alisol G
Catalog No.:BCN3461
CAS No.:155521-46-3
- 3a-Epiburchellin
Catalog No.:BCN7015
CAS No.:155551-61-4
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- VUF 10166
Catalog No.:BCC5060
CAS No.:155584-74-0
- Dehydroaglaiastatin
Catalog No.:BCN1699
CAS No.:155595-93-0
- PG-9 maleate
Catalog No.:BCC6779
CAS No.:155649-00-6
- Notoginsenoside Ft1
Catalog No.:BCN6434
CAS No.:155683-00-4
Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity.[Pubmed:18596648]
Molecules. 2008 Jun 1;13(6):1219-29.
Dragon's blood (Sangre de drago), a viscous red sap derived from Croton lechleri Muell-Arg (Euphorbiaceae), is extensively used by indigenous cultures of the Amazonian basin for its wound healing properties. The aim of this study was to identify the minor secondary metabolites and test the antioxidant activity of this sustance. A bioguided fractionation of the n-hexane, chloroform, n-butanol, and aqueous extracts led to the isolation of 15 compounds: three megastigmanes, four flavan-3-ols, three phenylpropanoids, three lignans, a clerodane, and the alkaloid taspine. In addition to these known molecules, six compounds were isolated and identified for the first time in the latex: blumenol B, blumenol C, 4,5-Dihydroblumenol A, erythro-guaiacyl-glyceryl-beta-O-4'- dihydroconiferyl ether, 2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-propane-1,3-diol and floribundic acid glucoside. Combinations of spectroscopic methods ((1)H-, (13)C- NMR and 2D-NMR experiments), ESI-MS, and literature comparisons were used for compound identification. In vitro antioxidant activities were assessed by DPPH, total antioxidant capacity and lipid peroxidation assays. Flavan-3-ols derivatives (as major phenolic compounds in the latex) exhibited the highest antioxidant activity.
[Chemical constituents from stems of Brucea mollis and their cytotoxic activity].[Pubmed:24199564]
Zhongguo Zhong Yao Za Zhi. 2013 Jul;38(14):2321-4.
Ten compounds were isolated from the stems of Brucea mollis by various chromatographic techniques such as column chromatography on silica gel and Sephadex LH-20, and preparative HPLC, and their structures were elucidated as deacetylated isobrucein B (1), indaquassin X (2), cleomiscosin A (3), cleomiscosin B (4), (+)-lyoniresinol (5), (+)-epipinoresinol(6), (+)-pinoresinol (7), (+)-syringaresinol (8), 4,5-Dihydroblumenol A (9) and adenosine (10) on the basis of spectroscopic data analysiS. All compounds were obtained from this plant for the first time, moreover, compound 1 was a new natural product. Compound 2 showed significant cytotoxic activities against the human cell lines HT-29, HepG2, BGC-823 and SKOV3 with IC50 values of 0.84-3.97 micromol x L(-1).


