Istradefylline (KW-6002)Selective A2A receptor antagonist CAS# 155270-99-8 |
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- Preladenant
Catalog No.:BCC1868
CAS No.:377727-87-2
- Pentostatin
Catalog No.:BCC1845
CAS No.:53910-25-1
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
- LUF6000
Catalog No.:BCC1710
CAS No.:890087-21-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 155270-99-8 | SDF | Download SDF |
| PubChem ID | 5311037 | Appearance | Powder |
| Formula | C20H24N4O4 | M.Wt | 384.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | KW-6002 | ||
| Solubility | DMSO : 25.33 mg/mL (65.89 mM; Need ultrasonic and warming) | ||
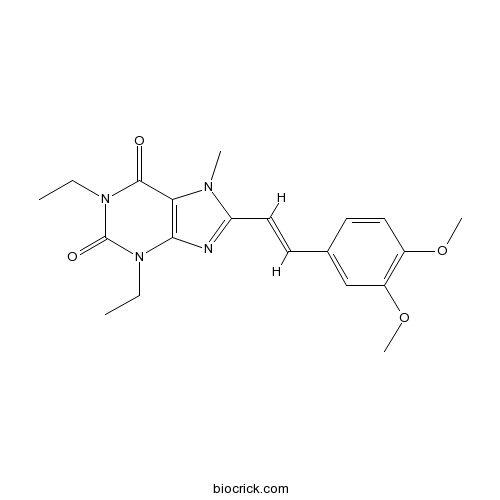
| Chemical Name | 8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methylpurine-2,6-dione | ||
| SMILES | CCN1C2=C(C(=O)N(C1=O)CC)N(C(=N2)C=CC3=CC(=C(C=C3)OC)OC)C | ||
| Standard InChIKey | IQVRBWUUXZMOPW-PKNBQFBNSA-N | ||
| Standard InChI | InChI=1S/C20H24N4O4/c1-6-23-18-17(19(25)24(7-2)20(23)26)22(3)16(21-18)11-9-13-8-10-14(27-4)15(12-13)28-5/h8-12H,6-7H2,1-5H3/b11-9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective adenosine A2A receptor antagonist (Ki values are 2.2 and 150 nM for A2A and A1 receptors respectively). Anticataleptic and antiparkinson agent; reverses drug-induced motor dysfunction in animal models. |

Istradefylline (KW-6002) Dilution Calculator

Istradefylline (KW-6002) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6013 mL | 13.0063 mL | 26.0125 mL | 52.0251 mL | 65.0313 mL |
| 5 mM | 0.5203 mL | 2.6013 mL | 5.2025 mL | 10.405 mL | 13.0063 mL |
| 10 mM | 0.2601 mL | 1.3006 mL | 2.6013 mL | 5.2025 mL | 6.5031 mL |
| 50 mM | 0.052 mL | 0.2601 mL | 0.5203 mL | 1.0405 mL | 1.3006 mL |
| 100 mM | 0.026 mL | 0.1301 mL | 0.2601 mL | 0.5203 mL | 0.6503 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KW-6002 is a selective adenosine A2A receptor antagonist, offering a novel mechanistic approach for the treatment of Parkinson’s disease (PD). A2A blockade will increase in GABAergic inhibition on the medium-sized neurons, leading to a net decrease in excessive activation of striatopallidal output.
In vitro: The affinity of KW-6002 for the A2AR is 70-fold greater than that for the A1 receptor. The binding affinities (Ki) of KW-6002 for human A1 receptor and A2A receptor are >287 nM and 9.12 nM, respectively [1].
In vivo: In MPTP neurotoxin model of PD in mice, KW-6002 significantly attenuated striatal dopamine depletion under various conditions. In addition, pretreatment with KW-6002 (3.3 mg/kg, i.p.) before a single dose of MPTP attenuated the partial dopamine and DOPAC depletions 1 week later [2].
Clinical trial: In a clinical study, the authors evaluated the safety and efficacy of KW-6002 40 mg as monotherapy for Parkinson’s disease (PD) in 176 patients. The primary efficacy outcome was changed from baseline to endpoint, while safe and well-tolerated, failed to show a significant improvement from placebo for this endpoint [1].
References:
[1] Park A, Stacy M. Istradefylline for the treatment of Parkinson's disease. Expert Opin Pharmacother. 2012 Jan;13(1):111-4.
[2] Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21(10):RC143.
- Peraksine
Catalog No.:BCN1694
CAS No.:15527-80-7
- Ritonavir
Catalog No.:BCC3620
CAS No.:155213-67-5
- Bimatoprost
Catalog No.:BCC4948
CAS No.:155206-00-1
- Methyl 7,15-dihydroxydehydroabietate
Catalog No.:BCN1693
CAS No.:155205-65-5
- 7alpha,15-Dihydroxydehydroabietic acid
Catalog No.:BCN7672
CAS No.:155205-64-4
- 4-(Dimethylamino)cinnamic acid
Catalog No.:BCN5031
CAS No.:1552-96-1
- Cinnamylideneacetic acid
Catalog No.:BCN7777
CAS No.:1552-94-9
- Cordifolioside A
Catalog No.:BCN8224
CAS No.:155179-20-7
- Physapruin A
Catalog No.:BCN7576
CAS No.:155178-03-3
- Plerixafor octahydrobromide
Catalog No.:BCC9123
CAS No.:155148-32-6
- Plerixafor 8HCl (AMD3100 8HCl)
Catalog No.:BCC4447
CAS No.:155148-31-5
- Rosiglitazone maleate
Catalog No.:BCC2262
CAS No.:155141-29-0
- NB-598 Maleate
Catalog No.:BCC1788
CAS No.:155294-62-5
- Alisol E 23-acetate
Catalog No.:BCN3459
CAS No.:155301-58-9
- (S)-Sulforaphane
Catalog No.:BCC8097
CAS No.:155320-20-0
- Fluconazole hydrate
Catalog No.:BCC4235
CAS No.:155347-36-7
- p-Menthane-1,3,8-triol
Catalog No.:BCN1695
CAS No.:155348-06-4
- Btk inhibitor 1 R enantiomer hydrochloride
Catalog No.:BCC5126
CAS No.:1553977-42-6
- Ethyl (E)-3'-hydroxy-4'-methoxycinnamate
Catalog No.:BCN3302
CAS No.:155401-23-3
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid methyl ester
Catalog No.:BCC8671
CAS No.:155405-80-4
- 4,5-Dihydroblumenol A
Catalog No.:BCN1696
CAS No.:155418-97-6
- JWH 015
Catalog No.:BCC5744
CAS No.:155471-08-2
- 3,6-Caryolanediol
Catalog No.:BCN1697
CAS No.:155485-76-0
- 1,9-Caryolanediol 9-acetate
Catalog No.:BCN1698
CAS No.:155488-34-9
Clinical efficacy of istradefylline (KW-6002) in Parkinson's disease: a randomized, controlled study.[Pubmed:20629136]
Mov Disord. 2010 Jul 30;25(10):1437-43.
The objectives of this study were to evaluate the efficacy of istradefylline at an oral dose of 20 mg or 40 mg once daily for 12 weeks in Parkinson's disease (PD) patients with motor complications on levodopa therapy based on the change in the daily OFF time compared with placebo and to assess the safety at these doses. A total of 363 subjects were randomly assigned to receive 20 mg/day istradefylline (n = 119), 40 mg/day istradefylline (n = 125), or placebo (n = 119). The primary outcome variable was the change from baseline at endpoint in daily OFF time based on patients' ON/OFF diaries. At endpoint, the daily OFF time reduced from baseline by 1.31 hours for 20 mg/day istradefylline (P = 0.013 as compared to the placebo), 1.58 hours for 40 mg/day istradefylline (P < 0.001), and 0.66 hours for placebo; istradefylline significantly reduced the daily OFF time compared with placebo. The UPDRS Part III subscale score (ON state) reduced by 5.7 at endpoint in both istradefylline groups and 3.7 in the placebo group (P = 0.006 for 20 mg/day and P = 0.006 for 40 mg/day group as compared with placebo). The most commonly reported drug-related treatment emergent adverse event (TEAE) was dyskinesia, which occurred in 2.5% (3/119) of subjects receiving placebo, 8.5% (10/118) receiving 20 mg/day istradefylline, and 6.4% (8/125) receiving 40 mg/day istradefylline. We conclude that istradefylline at 20 mg and 40 mg once daily is effective in relieving wearing-off fluctuations of PD patients. In addition, istradefylline was well tolerated at both doses.
Istradefylline for Parkinson's disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study.[Pubmed:22000279]
Parkinsonism Relat Disord. 2012 Feb;18(2):178-84.
BACKGROUND: Istradefylline (KW-6002) is a selective adenosine A(2A) receptor antagonist investigated as adjunctive therapy to levodopa in PD patients with motor response complications. In Phase 2b/3 studies, Istradefylline reduced OFF time without worsening troublesome dyskinesia and was well tolerated. METHODS: A randomized, 12-week, double-blind, placebo-controlled parallel-group study evaluated the efficacy of 10, 20, and 40 mg/day of Istradefylline in patients on levodopa therapy with motor response complications. The primary outcome measure was change from baseline to endpoint in the percentage of awake time/day spent in the OFF state as determined by patient diary. RESULTS: Six hundred and ten patients were randomized. Five hundred and eighty four patients were included in the Intent-to-treat (ITT) group-146 placebo patients and 149 in the 10 mg, 144 in the 20, and 145 patients in the 40 mg Istradefylline groups. Baseline demographics were similar between groups. Treatment cohorts had been diagnosed an average of 9 years diagnosis and 3.6 years from the onset of motor fluctuations; at baseline they had an average of 6.7 h of OFF time and an average UPDRS motor score of 22 when ON. At endpoint, the amount and percentage of OFF time did not differ between Istradefylline and placebo, however a dose-ordering response was observed. Changes from baseline in the UPDRS motor score in the on state for the 40 mg were modest but significant compared to placebo (2.9 vs. 0.8; p < 0.05). CONCLUSIONS: Although Istradefylline did not impact OFF time duration, it significantly improved motor score at 40 mg/day.
Antidepressant activity of the adenosine A2A receptor antagonist, istradefylline (KW-6002) on learned helplessness in rats.[Pubmed:24488405]
Psychopharmacology (Berl). 2014 Jul;231(14):2839-49.
RATIONALE: Istradefylline, an adenosine A2A receptor antagonist, improves motor function in animal models of Parkinson's disease (PD) and in patients with PD. In addition, some A2A antagonists exert antidepressant-like activity in rodent models of depression, such as the forced swim and the tail suspension tests. OBJECTIVE: We have investigated the effect of istradefylline on depression-like behaviors using the rat learned helplessness (LH) model. RESULTS: Acute, as well as chronic, oral administration of istradefylline significantly improved the inescapable shock (IES)-induced escape deficit with a degree of efficacy comparable to chronic treatment with the tricyclic antidepressant desipramine and the selective serotonin (5-HT) reuptake inhibitor, fluoxetine. Both the A1/A2A receptor nonspecific antagonist theophylline and the moderately selective antagonist CGS15943, but not the A1 selective antagonist DPCPX, ameliorated the IES-induced escape deficit. The enhancement of escape response by istradefylline was reversed by a local injection of the A2A specific agonist CGS21680 either into the nucleus accumbens, the caudate-putamen, or the paraventricular nucleus of the hypothalamus, but not by the A1 specific agonist R-PIA into the nucleus accumbens. Moreover, neither the 5-HT2A/2C receptor antagonist methysergide or the adrenergic alpha 2 antagonist yohimbine, nor the beta-adrenergic antagonist propranolol, affected the improvement of escape response induced by istradefylline. CONCLUSIONS: Istradefylline exerts antidepressant-like effects via modulation of A2A receptor activity which is independent of monoaminergic transmission in the brain. Istradefylline may represent a novel treatment option for depression in PD as well as for the motor symptoms.
Antidepressant-like activity of the adenosine A(2A) receptor antagonist, istradefylline (KW-6002), in the forced swim test and the tail suspension test in rodents.[Pubmed:24201052]
Pharmacol Biochem Behav. 2013 Dec;114-115:23-30.
RATIONALE: Depression is common in Parkinson's disease (PD) but its response to classical antidepressants is not clear. The adenosine A2A antagonist istradefylline is effective in the treatment of the motor symptoms of PD but inhibition of the adenosine A2A receptor may also induce antidepressant-like effects. OBJECTIVE: We have investigated whether istradefylline might be effective in treating depression in PD using the forced swimming test (FST) and the tail suspension test (TST) in rodents. RESULTS: Istradefylline significantly decreased immobility time in the FST in both rats and mice (0.16mg/kg and higher) with comparable efficacy to an equivalent dose of the tricyclic antidepressants, desipramine and imipramine. Both 8-OH-DPAT (5-HT1A agonist) and quinpirole (D2 agonist) also reduced the immobility time. The istradefylline-induced reduction of immobility time was attenuated by corticosterone. In addition, the combined use of a sub-threshold dose of istradefylline and the serotonin-noradrenaline reuptake inhibitor venlafaxine ameliorated depression-like behavior in the mouse FST. In the mouse TST, istradefylline (0.08mg/kg and higher) decreased immobility time. Moreover, co-administration of istradefylline with paroxetine or fluoxetine (selective serotonin reuptake inhibitors) or deprenyl (MAO-B inhibitor) at doses that did not show antidepressant-like effects when administered alone, resulted in a significant reduction in immobility time. CONCLUSIONS: Istradefylline alone or co-administered with currently available antidepressants, may be useful for the treatment of depression as well as motor symptoms of PD. Its effects might be, at least in part, attributable to modulation of hypothalamic-pituitary-adrenal axis.
Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP.[Pubmed:10591873]
Psychopharmacology (Berl). 1999 Nov;147(1):90-5.
RATIONALE: Current treatment of Parkinson's disease (PD) is based on dopamine replacement therapy, but this leads to long term complications, including dyskinesia. Adenosine A2A receptors are particularly abundant in the striatum and would be a target for an alternative approach to the treatment of PD. OBJECTIVES: The purpose of this study is to examine the efficacy and potency of the novel selective adenosine A2A receptor antagonist (E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dhydro- 1H-purine-2,6- dione (KW-6002) in ameliorating the motor deficits in various mouse models of Parkinson's disease. METHODS: We evaluated the efficacy and potency of KW-6002 and other reference compounds in the selective adenosine A2A receptor agonist 2-[p-(2-carboxyethyl)phenethylamino]-5'-N-ethylcarboxamidoadenosin e (CGS 21680)-, haloperidol- or reserpine-induced catalepsy models. The effect of KW-6002 on reserpine or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride(MPTP)-induced hypolocomotion was also examined. RESULTS: The ED50s of KW-6002 in the reversal of CGS21680-induced and reserpine-induced catalepsy were 0.05 mg/kg, PO and 0.26 mg/kg, PO, respectively. Compared to the ED50 of other adenosine antagonists and dopamine agonist drugs, KW-6002 is over 10 times as potent in these models. KW-6002 also ameliorated the hypolocomotion (minimum effective dose; 0.16 mg/kg) induced by nigral dopaminergic dysfunction with MPTP or reserpine treatment. Combined administrations of subthreshold doses of KW-6002 and L-dopa (50 mg/kg, PO) exerted prominent effects on haloperidol-induced and reserpine-induced catalepsy, suggesting that there may be a synergism between the adenosine A2A receptor antagonist KW-6002 and dopaminergic agents. CONCLUSIONS: To our knowledge, KW-6002 is the most potent and orally active adenosine A2A receptor antagonist in experimental models of Parkinson's disease, and may offer a new therapeutic approach to the treatment of Parkinson's disease.
Adenosine A2A receptors modify motor function in MPTP-treated common marmosets.[Pubmed:9760134]
Neuroreport. 1998 Aug 24;9(12):2857-60.
Both adenosine A1 and A2 receptor populations are located in the striatum and can modify locomotor activity, and they may form a therapeutic target for Parkinson's disease (PD). Administration of the selective adenosine A2A antagonist (E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dihydro-1H-pu rine-2,6-dione (KW-6002) to MPTP-treated common marmosets increased locomotor activity. In contrast, administration of the selective A1 receptor antagonist 1,3-dipropyl-8-cyclopentylxantine (DPCPX) had no effect on locomotion. Administration of the adenosine A2A receptor agonist 2-[p-[2-(2-aminoethylamino) carbonylethyl] phenethyl amino]-5'-N-ethylcarboxamidoadenosine (APEC) dose dependently suppressed basal locomotor activity. A minimally effective dose of APEC (0.62 mg/kg, i.p) completely reversed the increase in locomotor activity produced by administration of KW-6002. The adenosine A2A receptor appears to be an important target for the treatment of basal ganglia disorders, particularly PD.


