CGS 21680Adenosine A2 receptor agonists,potent and selective CAS# 120225-54-9 |
- CGH 2466 dihydrochloride
Catalog No.:BCC7338
CAS No.:1177618-54-0
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- Preladenant
Catalog No.:BCC1868
CAS No.:377727-87-2
- ANR 94
Catalog No.:BCC7815
CAS No.:634924-89-3
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120225-54-9 | SDF | Download SDF |
| PubChem ID | 104924 | Appearance | Powder |
| Formula | C23H29N7O6 | M.Wt | 499.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >19.3mg/mL in DMSO | ||
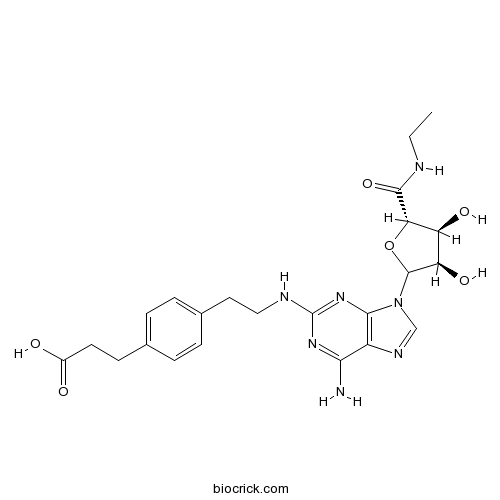
| Chemical Name | 3-[4-[2-[[6-amino-9-[(3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid | ||
| SMILES | CCNC(=O)C1C(C(C(O1)N2C=NC3=C2N=C(N=C3N)NCCC4=CC=C(C=C4)CCC(=O)O)O)O | ||
| Standard InChIKey | PAOANWZGLPPROA-OFRRTHGGSA-N | ||
| Standard InChI | InChI=1S/C23H29N7O6/c1-2-25-21(35)18-16(33)17(34)22(36-18)30-11-27-15-19(24)28-23(29-20(15)30)26-10-9-13-5-3-12(4-6-13)7-8-14(31)32/h3-6,11,16-18,22,33-34H,2,7-10H2,1H3,(H,25,35)(H,31,32)(H3,24,26,28,29)/t16-,17+,18-,22?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CGS 21680 is agonist of A2A adenosine receptor with Ki value of 27 nM. | |||||
| Targets | A2A adenosine receptor | |||||
| IC50 | 27 nM (Ki) | |||||

CGS 21680 Dilution Calculator

CGS 21680 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0019 mL | 10.0096 mL | 20.0192 mL | 40.0384 mL | 50.048 mL |
| 5 mM | 0.4004 mL | 2.0019 mL | 4.0038 mL | 8.0077 mL | 10.0096 mL |
| 10 mM | 0.2002 mL | 1.001 mL | 2.0019 mL | 4.0038 mL | 5.0048 mL |
| 50 mM | 0.04 mL | 0.2002 mL | 0.4004 mL | 0.8008 mL | 1.001 mL |
| 100 mM | 0.02 mL | 0.1001 mL | 0.2002 mL | 0.4004 mL | 0.5005 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CGS 21680 is a selective agonists of A2 adenosine receptor with IC50 value of 22nM [1].
CGS 21680 is found to be potent and selective adenosine A2 receptor agonists. In a radioligand binding in vitro assay, CGS 21680 binds A2 receptor with IC50 value of 22nM and the A1/A2 ratio is 141, In the perfused rat heart model, CGS 21680 can increase coronary flow with greater EC25 value than 1000nM. It shows a good separation between its ability to induce vasorelaxation and bradycardia. CGS 21680 also has potent effect on blood pressure in vivo with EC25 value of 9µg/kg and it can induce an increase in heart rate [1].
It is reported that CGS 21680 also has the potency of anti-inflammatory. It can reduce the development of acute lung inflammation in a mouse model of carrageenan-induced pleurisy. In both prior and post-treatment, CGS 21680 can reduce the number of inflammatory cells and the degree of lung injury [2].
References:
[1] Alan J. Hutchison, Randy L. Webb, Howard H. Oei, Geetha R. Ghai, Mark B. Zimmerman and Michael Williams. CGS 21680C, an A2 Selective Adenosine Receptor Agonist with Preferential Hypotensive Activity. The Journal of Pharmacology and Experimental Therapeutics. 1989, 25 (1): 47-55.
[2] Daniela Impellizzeri, Rosanna Di Paola, Emanuela Esposito, Emanuela Mazzon, Irene Paterniti, Alessia Melani, Placido Bramanti, Felicita Pedata, Salvatore Cuzzocrea. CGS 21680, an agonist of the adenosine (A2A) receptor, decreases acute lung inflammation. European Journal of Pharmacology. 2011, 68: 305-316.
- 1beta-Hydroxyeuscaphic acid
Catalog No.:BCN3517
CAS No.:120211-98-5
- Tenidap
Catalog No.:BCC7419
CAS No.:120210-48-2
- NMS-P715
Catalog No.:BCC6373
CAS No.:1202055-34-2
- Clopidogrel Related Compound C
Catalog No.:BCN2689
CAS No.:120202-71-3
- Clopidogrel
Catalog No.:BCC2497
CAS No.:120202-66-6
- 3,4-Dihydroxycinnamamide
Catalog No.:BCN6090
CAS No.:1202-41-1
- Cynoglossamine
Catalog No.:BCN1970
CAS No.:120193-39-7
- TCS 2210
Catalog No.:BCC7798
CAS No.:1201916-31-5
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- Vinflunine Tartrate
Catalog No.:BCC4602
CAS No.:1201898-17-0
- Crotaleschenine
Catalog No.:BCN2077
CAS No.:120154-95-2
- IPI-145 (INK1197)
Catalog No.:BCC1104
CAS No.:1201438-56-3
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- CNX-774
Catalog No.:BCC4394
CAS No.:1202759-32-7
- Salvinolone
Catalog No.:BCN3215
CAS No.:120278-22-0
- 4-Hydroxysapriparaquinone
Catalog No.:BCN4806
CAS No.:120278-25-3
- Dorzolamide
Catalog No.:BCC4287
CAS No.:120279-96-1
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- Citroside A
Catalog No.:BCN7294
CAS No.:120330-44-1
- DASA-58
Catalog No.:BCC6522
CAS No.:1203494-49-8
- Cyclotraxin B
Catalog No.:BCC6357
CAS No.:1203586-72-4
- AS 1949490
Catalog No.:BCC7762
CAS No.:1203680-76-5
- Jionoside B1
Catalog No.:BCN2858
CAS No.:120406-37-3
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
Differential effects of the adenosine A(2)A agonist CGS-21680 and haloperidol on food-reinforced fixed ratio responding in the rat.[Pubmed:21898173]
Psychopharmacology (Berl). 2012 Mar;220(1):205-13.
RATIONALE: Previous studies have shown that adenosine A(2A) receptors are colocalized with dopamine D(2) receptors on striatal neurons. Activation of these two receptors has antagonistic effects under a number of conditions suggesting that stimulation of adenosine A(2A) receptors may have behavioral effects resembling those produced by blockade of dopamine D(2) receptors, but this possibility has been investigated in a limited number of situations. OBJECTIVE: We compared the effects of the adenosine A(2A) agonist CGS-21680 and the preferential D(2) dopamine antagonist haloperidol in a situation in which dopamine blockade produces a distinctive pattern of behavioral effects. MATERIALS AND METHODS: Six rats were trained to lever press for food reward on a fixed ratio 15 schedule of reinforcement and then tested after being injected with various doses of CGS-21680 (0.064, 0.128, and 0.25 mg/kg) and haloperidol (0.25 and 0.1 mg/kg). RESULTS: Haloperidol produced a dose-dependent suppression of lever pressing with mean response rates declining across the duration of the test session. CGS-21680 also produced a dose-dependent suppression of responding, but this effect was not temporally graded, and responding was equivalently suppressed across the duration of the session. Additionally, CGS-21680 increased post-reinforcement pause duration to a much greater extent than did haloperidol. CONCLUSIONS: On this task, the behavioral effects of CGS-21680 do not resemble those produced by haloperidol. Several explanations of this discrepancy are possible, the most likely being that the observed behavioral effects of CGS-21680 result from an action at a site other than D(2) receptor-expressing striatal neurons.
The adenosine A2A receptor agonist CGS 21680 decreases ethanol self-administration in both non-dependent and dependent animals.[Pubmed:23301633]
Addict Biol. 2013 Sep;18(5):812-25.
There is emerging evidence that the adenosinergic system might be involved in drug addiction and alcohol dependence. We have already demonstrated the involvement of A2A receptors (A2AR) in ethanol-related behaviours in mice. Here, we investigated whether the A2AR agonist CGS 21680 can reduce ethanol operant self-administration in both non-dependent and ethanol-dependent Wistar rats. To rule out a potential involvement of the A1R in the effects of CGS 21680, we also tested its effectiveness to reduce ethanol operant self-administration in both heterozygous and homozygous A1R knockout mice. Our results demonstrated that CGS 21680 (0.065, 0.095 and 0.125 mg/kg, i.p.) had a bimodal effect on 10% ethanol operant self-administration in non-dependent rats. The intermediate dose was also effective in reducing 2% sucrose self-administration. Interestingly, the intermediate dose reduced 10% ethanol self-administration in dependent animals more effectively (75% decrease) when compared with non-dependent animals (57% decrease). These results suggest that the A2AR are involved in CGS 21680 effects since the reduction of ethanol self-administration was not dependent upon the presence of A1R in mice. In conclusion, our findings demonstrated the effectiveness of the A2AR agonist CGS 21680 in a preclinical model of alcohol addiction and suggested that the adenosinergic pathway is a promising target to treat alcohol addiction.
Topical application of the adenosine A2A receptor agonist CGS-21680 prevents phorbol-induced epidermal hyperplasia and inflammation in mice.[Pubmed:24889129]
Exp Dermatol. 2014 Aug;23(8):555-60.
The nucleoside adenosine is a known regulator of immunity and inflammation that mediates, at least in part, the anti-inflammatory effect of methotrexate, an immunosuppressive agent widely used to treat autoimmune inflammatory diseases. Adenosine A2A receptors play a key role in the inhibition of the inflammatory process besides promoting wound healing. Therefore, we aimed to determine the topical effect of a selective agonist, CGS-21680, on a murine model of skin hyperplasia with a marked inflammatory component. Pretreatment with either CGS-21680 (5 mug per site) or the reference agent dexamethasone (200 mug/site) prevented the epidermal hyperplasia and inflammatory response induced by topical application of 12-O-tetradecanoylphorbol-13-acetate (TPA, 2 nmol/site) for three consecutive days. The histological analysis showed that both CGS-21680 and dexamethasone produced a marked reduction of inflammatory cell infiltrate, which correlated with diminished myeloperoxidase (MPO) activity in skin homogenates. Both treatments reduced the levels of the chemotactic mediators LTB4 and CXCL-1, and the inflammatory cytokine TNF-alpha, through the suppression of NFkappaB phosphorylation. The immunohistochemical analysis of the hyperproliferative markers cytokeratin 6 (CK6) and Ki67 revealed that while both agents inhibit the number of proliferating cells in the epidermis, CGS-21680 treatment promoted dermal fibroblasts proliferation. Consistently, increased collagen deposition in dermis was observed in tissue sections from agonist-treated mice. Our results showed that CGS 21680 efficiently prevents phorbol-induced epidermal hyperplasia and inflammation in mice without the deleterious atrophic effect of topical corticosteroids.
CGS 21680, an agonist of the adenosine (A2A) receptor, reduces progression of murine type II collagen-induced arthritis.[Pubmed:21765105]
J Rheumatol. 2011 Oct;38(10):2119-29.
OBJECTIVE: The aim of our study was to investigate the effect of an adenosine A2A receptor agonist, 2-[p-(2-carboxyethyl)phenylethylamino]-50 ethylcarboxamidoadenosine (CGS 21680), on modulation of the inflammatory response in mice subjected to collagen-induced arthritis (CIA). METHODS: CIA was induced by intradermal injection of 100 mul of emulsion containing 100 mug of bovine type II collagen (CII) and complete Freund's adjuvant (CFA) at the base of the tail. On Day 21, a second injection of CII in CFA was administered. Immunized mice developed erosive hind paw arthritis. Macroscopic clinical evidence of CIA first appeared as periarticular erythema and edema in the hind paws. The incidence of CIA was 100% by Day 27 in the CII challenged mice and the severity of CIA progressed over a 35-day period, with radiographic evaluation revealing focal resorption of bone. The histopathology of CIA included erosion of cartilage at the joint margins. RESULTS: Treatment of mice with CGS 21680 starting at the onset of arthritis (Day 25) ameliorated the clinical signs at Days 26-35 and improved histological status in the joint and paw. The degree of oxidative and nitrosative damage was significantly reduced in CGS 21680-treated mice as indicated by elevated levels of malondialdehyde, formation of nitrotyrosine, and activation of poly(ADP-ribose) polymerase. Plasma levels of proinflammatory cytokines such as tumor necrosis factor, interleukin 1ss (IL-1ss) and IL-6 were also reduced by CGS 21680. Treatment with CGS 21680 also decreased the expression of inducible nitric oxide synthase and cyclooxygenase-2. CONCLUSION: We demonstrate that CGS 21680 exerts an antiinflammatory effect during chronic inflammation and ameliorates the tissue damage associated with CIA.


