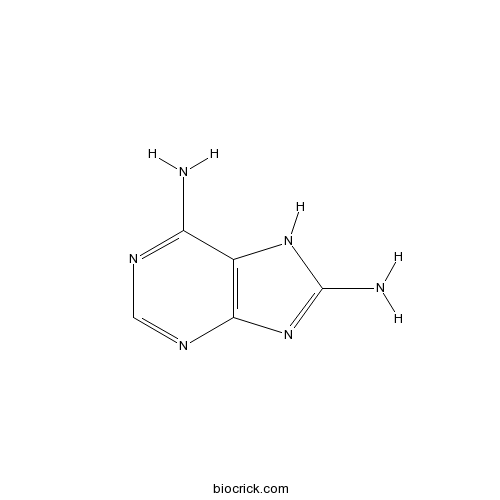
8-AminoadenineAdenine receptor agonist CAS# 28128-33-8 |
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28128-33-8 | SDF | Download SDF |
| PubChem ID | 4371597 | Appearance | Powder |
| Formula | C5H6N6 | M.Wt | 150.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 2eq.HCl and to 50 mM in DMSO | ||
| Chemical Name | 7H-purine-6,8-diamine | ||
| SMILES | C1=NC2=C(C(=N1)N)NC(=N2)N | ||
| Standard InChIKey | PFUVOLUPRFCPMN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H6N6/c6-3-2-4(9-1-8-3)11-5(7)10-2/h1H,(H5,6,7,8,9,10,11) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Adenine receptor agonist (Ki = 0.0341 μM in HEK293 cells expressing an adenine binding site). Displays 190-fold increased potency at the human binding site over the rat adenine receptor (rAde1R) (Ki = 6.51 μM). |

8-Aminoadenine Dilution Calculator

8-Aminoadenine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6605 mL | 33.3023 mL | 66.6045 mL | 133.209 mL | 166.5113 mL |
| 5 mM | 1.3321 mL | 6.6605 mL | 13.3209 mL | 26.6418 mL | 33.3023 mL |
| 10 mM | 0.666 mL | 3.3302 mL | 6.6605 mL | 13.3209 mL | 16.6511 mL |
| 50 mM | 0.1332 mL | 0.666 mL | 1.3321 mL | 2.6642 mL | 3.3302 mL |
| 100 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.3321 mL | 1.6651 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
8-Aminoadenine is an agonist of adenine receptor with Ki value of 0.0341 μM [1].
Adenine receptor is a G protein-coupled receptor activated by the nucleoside adenosine. There are four types of adenosine receptors: A1, A2A, A2B and A3. A1 and A2A receptors play an important role in the heart, regulating coronary blood flow and myocardial oxygen consumption. A2B and A3 receptors are involved in inflammation and immune responses.
8-Aminoadenine is an adenine receptor agonist with Ki values of 0.0341 and 6.51 μM for human and rat, respectively. In HEK293 cell membrane, 8-Aminoadenine was more potent than adenine (Ki = 0.0471 μM) and exhibited 190-fold more potent than the rat binding site. In 1321N1 astrocytoma cells expressing the rAde1R, 8-Aminoadenine (500 μM) inhibited isoproterenol-stimulated cAMP accumulation, which suggested that 8-Aminoadenine inhibited adenine uptake [1].
Reference:
[1]. Borrmann T, Abdelrahman A, Volpini R, et al. Structure-activity relationships of adenine and deazaadenine derivatives as ligands for adenine receptors, a new purinergic receptor family. J Med Chem, 2009, 52(19): 5974-5989.
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
- Adamantane
Catalog No.:BCN8481
CAS No.:281-23-2
- Chaetocin
Catalog No.:BCC2429
CAS No.:28097-03-2
- (+)-Ulopterol
Catalog No.:BCN1228
CAS No.:28095-18-3
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- Piperazine Ferulate
Catalog No.:BCN3277
CAS No.:171876-65-6
- 2,6-Bis(2-benzimidazolyl)pyridine
Catalog No.:BCC8504
CAS No.:28020-73-7
- 25-O-methylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1464
CAS No.:27994-13-4
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
- JTE 907
Catalog No.:BCC7380
CAS No.:282089-49-0
- Phlorin
Catalog No.:BCN5177
CAS No.:28217-60-9
- HOOBt
Catalog No.:BCC2817
CAS No.:28230-32-2
- Reynosin
Catalog No.:BCN5178
CAS No.:28254-53-7
- Tyrphostin A1
Catalog No.:BCC5404
CAS No.:2826-26-8
Property editing of peptide nucleic acids (PNA): gem-dimethyl, cyanuryl and 8-aminoadenine PNAs.[Pubmed:18029564]
Nucleic Acids Symp Ser (Oxf). 2007;(51):17-8.
We herein describe the introduction of gem-dimethyl substitution into the aminoethylglycyl backbone of PNA to impart steric constraint and pre-organise PNA for selective recognition of nucleic acids. Introduction of cyanuric acid and 8-Aminoadenine as pyrimidine and purine analogs that can form base pairing from either face is also described to overcome the rotameric problems in PNA sidechain orientations and thereby enhance the statistical probability for base pairing. The UV-thermal melting studies of the derived triplexes with complementary DNA provide support for this rationale.
Structure-activity relationships of adenine and deazaadenine derivatives as ligands for adenine receptors, a new purinergic receptor family.[Pubmed:19731917]
J Med Chem. 2009 Oct 8;52(19):5974-89.
Adenine derivatives bearing substituents in the 2-, N(6)-, 7-, 8-, and/or 9-position and a series of deazapurines were synthesized and investigated in [(3)H]adenine binding studies at the adenine receptor in rat brain cortical membrane preparations (rAde1R). Steep structure-activity relationships were observed. Substitution in the 8-position (amino, dimethylamino, piperidinyl, piperazinyl) or in the 9-position (2-morpholinoethyl) with basic residues or introduction of polar substituents at the 6-amino function (hydroxy, amino, acetyl) represented the best modifications. Functional evaluation of selected adenine derivatives in adenylate cyclase assays at 1321N1 astrocytoma cells stably expressing the rAde1R showed that all compounds investigated were agonists or partial agonists. A subset of compounds was additionally investigated in binding studies at human embryonic kidney (HEK293) cells, which also express a high-affinity adenine binding site. Structure-affinity relationships at the human cell line were similar to those at the rAde1R, but not identical. In particular, N(6)-acetyladenine (25, K(i) rat: 2.85 microM; K(i) human: 0.515 microM) and 8-Aminoadenine (33, K(i) rat: 6.51 microM; K(i) human: 0.0341 microM) were much more potent at the human as compared to the rat binding site. The new AdeR ligands may serve as lead structures and contribute to the elucidation of the functions of the adenine receptor family.


