SB 216763GSK-3 inhibitor,ATP-competitive,potent and selective CAS# 280744-09-4 |
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- TWS119
Catalog No.:BCC4512
CAS No.:601514-19-6
- GSK-3 inhibitor 1
Catalog No.:BCC4126
CAS No.:603272-51-1
- AZD1080
Catalog No.:BCC4508
CAS No.:612487-72-6
- GSK-3 Inhibitor IX (BIO)
Catalog No.:BCC4510
CAS No.:667463-62-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 280744-09-4 | SDF | Download SDF |
| PubChem ID | 176158 | Appearance | Powder |
| Formula | C19H12Cl2N2O2 | M.Wt | 371.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (269.38 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
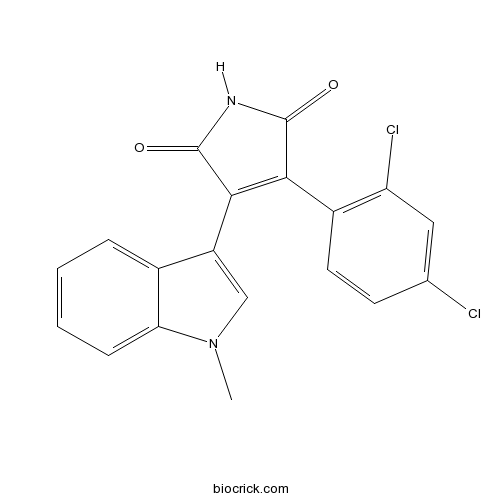
| Chemical Name | 3-(2,4-dichlorophenyl)-4-(1-methylindol-3-yl)pyrrole-2,5-dione | ||
| SMILES | CN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=C(C=C(C=C4)Cl)Cl | ||
| Standard InChIKey | JCSGFHVFHSKIJH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective, ATP-competitive glycogen synthase kinase-3 (GSK-3) inhibitor (IC50 = 34.3 nM for GSK-3α). Equally effective at inhibiting human GSK-3α and GSK-3β. Exhibits minimal activity against 24 other protein kinases (IC50 >10 μM). Stimulates glycogen synthesis in liver cells, and induces β-catenin-dependent gene transcription. Neuroprotective; also reduces pulmonary inflammation and fibrosis in a mouse model. Shown to maintain mouse embryonic stem cells in a pluripotent state. |

SB 216763 Dilution Calculator

SB 216763 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6938 mL | 13.4691 mL | 26.9382 mL | 53.8764 mL | 67.3455 mL |
| 5 mM | 0.5388 mL | 2.6938 mL | 5.3876 mL | 10.7753 mL | 13.4691 mL |
| 10 mM | 0.2694 mL | 1.3469 mL | 2.6938 mL | 5.3876 mL | 6.7346 mL |
| 50 mM | 0.0539 mL | 0.2694 mL | 0.5388 mL | 1.0775 mL | 1.3469 mL |
| 100 mM | 0.0269 mL | 0.1347 mL | 0.2694 mL | 0.5388 mL | 0.6735 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SB-216763 is a Potent and selective inhibitor of glycogen synthase kinase-3 (GSK-3) with IC50 of 34.3 nM. It competes with ATP and potently inhibits the activity against both GSK-3α and GSK-3β.
GSK-3, one of several known substrates of PKB, is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues.
SB-216763 treatment can protect neurones of both central and peripheral nervous system from cell death which is mediated by PI3K pathway ex vivo [1]. This component was also shown to maintain the pluripotency of mouse embryonic stem cells (mESCs) without exogenous leukemia inhibitory factor (LIF) when cultured on mouse embryonic fibroblasts (MEFs) [2].
The component has also been used extensively in various animal models to study the role of GSK-3. In a bleomycin-induced mice lung fibrosis model, SB-216763 administration protected mice from lung inflammation and the subsequent fibrosis, which was associated with decreased production of inflammatory cytokine by macrophages [3]. Besides, treatment of SB-216763 reduced infarct size and prevented cardiac ischemia in a model of regional myocardial ischemia-reperfusion in rats [4].
References:
1. Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem 2001,77:94-102.
2. Kirby LA, Schott JT, Noble BL, Mendez DC, Caseley PS, Peterson SC, et al. Glycogen synthase kinase 3 (GSK3) inhibitor, SB-216763, promotes pluripotency in mouse embryonic stem cells. PLoS One 2012,7:e39329.
3. Gurrieri C, Piazza F, Gnoato M, Montini B, Biasutto L, Gattazzo C, et al. 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763), a glycogen synthase kinase-3 inhibitor, displays therapeutic properties in a mouse model of pulmonary inflammation and fibrosis. J Pharmacol Exp Ther 2010,332:785-794.
4. Obame FN, Plin-Mercier C, Assaly R, Zini R, Dubois-Rande JL, Berdeaux A, et al. Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3 beta, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J Pharmacol Exp Ther 2008,326:252-258.
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- Piperazine Ferulate
Catalog No.:BCN3277
CAS No.:171876-65-6
- 2,6-Bis(2-benzimidazolyl)pyridine
Catalog No.:BCC8504
CAS No.:28020-73-7
- 25-O-methylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1464
CAS No.:27994-13-4
- Cimigenoside
Catalog No.:BCN5174
CAS No.:27994-11-2
- H-Cys(Trt)-OH
Catalog No.:BCC2911
CAS No.:2799-07-7
- Bellidifolin
Catalog No.:BCN7424
CAS No.:2798-25-6
- Gardenin B
Catalog No.:BCN3816
CAS No.:2798-20-1
- Isosteviol
Catalog No.:BCN2685
CAS No.:27975-19-5
- 1,6-Dibromopyrene
Catalog No.:BCC8428
CAS No.:27973-29-1
- 2-Amino-3-chloro-1,4-naphthoquinone
Catalog No.:BCC8525
CAS No.:2797-51-5
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- (+)-Ulopterol
Catalog No.:BCN1228
CAS No.:28095-18-3
- Chaetocin
Catalog No.:BCC2429
CAS No.:28097-03-2
- Adamantane
Catalog No.:BCN8481
CAS No.:281-23-2
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
Glycogen synthase kinase 3 (GSK3) inhibitor, SB-216763, promotes pluripotency in mouse embryonic stem cells.[Pubmed:22745733]
PLoS One. 2012;7(6):e39329.
Canonical Wnt/beta-catenin signaling has been suggested to promote self-renewal of pluripotent mouse and human embryonic stem cells. Here, we show that SB-216763, a glycogen synthase kinase-3 (GSK3) inhibitor, can maintain mouse embryonic stem cells (mESCs) in a pluripotent state in the absence of exogenous leukemia inhibitory factor (LIF) when cultured on mouse embryonic fibroblasts (MEFs). MESCs maintained with SB-216763 for one month were morphologically indistinguishable from LIF-treated mESCs and expressed pluripotent-specific genes Oct4, Sox2, and Nanog. Furthermore, Nanog immunostaining was more homogenous in SB-216763-treated colonies compared to LIF. Embryoid bodies (EBs) prepared from these mESCs expressed early-stage markers for all three germ layers, and could efficiently differentiate into cardiac-like cells and MAP2-immunoreactive neurons. To our knowledge, SB-216763 is the first GSK3 inhibitor that can promote self-renewal of mESC co-cultured with MEFs for more than two months.
Inhibition of glycogen synthase kinase-3 by SB 216763 affects acquisition at lower doses than expression of amphetamine-conditioned place preference in rats.[Pubmed:27984209]
Behav Pharmacol. 2017 Jun;28(4):262-271.
Dopamine (DA) drives incentive learning, whereby neutral stimuli acquire the ability to elicit responses. DA influences the signaling molecule glycogen synthase kinase-3 (GSK3). Inhibition of GSK3 attenuates the development of behavioral sensitization to stimulant drugs and conditioned place preference (CPP), a measure of incentive learning. We examined the role of GSK3 in the nucleus accumbens (NAc) of rats in CPP produced by amphetamine (1.5 mg/kg, i.p. or 20.0 mug/0.5 mul/side intra-NAc) by administering the inhibitor SB 216763 (1.0, 2.0, and 2.5 mg/kg, i.p. or 0.03, 0.30, 3.00, and 5.00 mug/0.5 mul/side intra-NAc) during acquisition or expression. We hypothesized a dose-dependent effect of SB 216763 and that acquisition would be affected by smaller doses than expression. For the systemic groups, 1.0 mg/kg of SB 216763 did not block CPP; 2.0 mg/kg administered in acquisition but not expression blocked CPP; and 2.5 mg/kg administered in either phase blocked CPP. For the central groups, 0.03 mug/0.5 mul/side of SB 216763 prevented acquisition but not expression, whereas larger doses administered in either phase blocked CPP. Thus, systemic or NAc inhibition of GSK3 by SB 216763 during acquisition or expression blocks amphetamine-produced CPP and acquisition is sensitive to lower doses than expression.
Synthesis and Initial in Vivo Studies with [(11)C]SB-216763: The First Radiolabeled Brain Penetrative Inhibitor of GSK-3.[Pubmed:26005531]
ACS Med Chem Lett. 2015 Mar 10;6(5):548-52.
Quantifying glycogen synthase kinase-3 (GSK-3) activity in vivo using positron emission tomography (PET) imaging is of interest because dysregulation of GSK-3 is implicated in numerous diseases and neurological disorders for which GSK-3 inhibitors are being considered as therapeutic strategies. Previous PET radiotracers for GSK-3 have been reported, but none of the published examples cross the blood-brain barrier. Therefore, we have an ongoing interest in developing a brain penetrating radiotracer for GSK-3. To this end, we were interested in synthesis and preclinical evaluation of [(11)C]SB-216763, a high-affinity inhibitor of GSK-3 (K i = 9 nM; IC50 = 34 nM). Initial radiosyntheses of [(11)C]SB-216763 proved ineffective in our hands because of competing [3 + 3] sigmatropic shifts. Therefore, we have developed a novel one-pot two-step synthesis of [(11)C]SB-216763 from a 2,4-dimethoxybenzyl-protected maleimide precursor, which provided high specific activity [(11)C]SB-216763 in 1% noncorrected radiochemical yield (based upon [(11)C]CH3I) and 97-100% radiochemical purity (n = 7). Initial preclinical evaluation in rodent and nonhuman primate PET imaging studies revealed high initial brain uptake (peak rodent SUV = 2.5 @ 3 min postinjection; peak nonhuman primate SUV = 1.9 @ 5 min postinjection) followed by washout. Brain uptake was highest in thalamus, striatum, cortex, and cerebellum, areas known to be rich in GSK-3. These results make the arylindolemaleimide skeleton our lead scaffold for developing a PET radiotracer for quantification of GSK-3 density in vivo and ultimately translating it into clinical use.
[Effects of glycogen synthase kinase 3beta overexpression in rat and glycogen synthase kinase 3beta inhibitor SB-216763 on proliferation of hepatic oval cells].[Pubmed:23302485]
Zhonghua Wai Ke Za Zhi. 2012 Nov;50(11):1003-6.
OBJECTIVE: To research the effects of glycogen synthase kinase (GSK3beta) overexpression and GSK3beta inhibitor SB-216763 on the proliferation of hepatic oval cells in rats and its regulatory mechanisms by Wnt signaling pathway. METHODS: The hepatic oval cells WBF-344 were divided into the blank control group, GSK3beta over-expression group, DMSO control group and GSK3beta inhibitor groups, while the inhibitor groups set up three concentration gradients, that was 1, 5, 10 micromol/L. Using the GSK3beta over-expression lentivirus, which had been identified correctly, and SB-216763 dealt with the cells WBF-344. The cells morphology of each group was observed under the phase contrast inverted microscope, and the expression of fluorescence in the lentivirus-transfected group was observed under the fluorescent microscope. The proliferation of each group cells was tested by CCK8 kits. The cells' apoptosis was detected by AnnexinV-FITC/PI kits. The expression of GSK3beta, beta-catenin and cyclin D1 were detected by Western blot. RESULTS: The cells of GSK3beta over-expression group were fewer and obvious aging. However, in each inhibitor added group, the cells' division and proliferation was vigorous, and the condition was good. Moreover, the cells' proliferation was getting stronger with the concentration of SB-216763 increasing. A large number of green fluorescence was expressed in the lentivirus-transfected cells. The cells' proliferation in GSK3beta over-expression group restrained (t = 7.178, P < 0.01, as compared with control), while the cells' proliferation was vigorous in inhibitor groups (F = 45.030, P < 0.01, as compared with control). Flow Cytometry showed that the cells apoptosis was significant in GSK3beta over-expression group. Western blot showed that the expression of GSK3beta was increased, while the expression of beta-catenin and cyclin D1 was decreased in the over-expression group. The expression of GSK3beta had no significant difference among the control group and inhibitor groups. However, the expression of beta-catenin and cyclin D1 was significantly increased with the concentration of SB-216763 increasing. CONCLUSIONS: The overexpression of GSK3beta can inhibit the Wnt signaling pathway, thus restrain the cells' proliferation and promotes apoptosis. SB-216763 can activate the Wnt pathway, thus promotes cells' proliferation.
3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763), a glycogen synthase kinase-3 inhibitor, displays therapeutic properties in a mouse model of pulmonary inflammation and fibrosis.[Pubmed:19959748]
J Pharmacol Exp Ther. 2010 Mar;332(3):785-94.
Glycogen synthase kinase (GSK)-3 modulates the production of inflammatory cytokines. Because bleomycin (BLM) causes lung injury, which is characterized by an inflammatory response followed by a fibrotic degeneration, we postulated that blocking GSK-3 activity with a specific inhibitor could affect the inflammatory and profibrotic cytokine network generated in the BLM-induced process of pulmonary inflammation and fibrosis. Thus, here we investigated the effects of the GSK-3 inhibitor 3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione (SB216763) on a BLM-induced lung fibrosis model in mice. SB216763 prevented lung inflammation and the subsequent fibrosis when coadministered with BLM. Bronchoalveolar lavage fluid analysis of mice treated with BLM plus SB216763 revealed a significant reduction in BLM-induced alveolitis. Furthermore, SB216763 treatment was associated with a significantly lower production of inflammatory cytokines by macrophages. BLM-treated mice that received SB216763 developed alveolar epithelial cell damage and pulmonary fibrosis to a significantly lower extent compared with BLM-treated controls. These findings suggest that GSK-3 inhibition has a protective effect on lung fibrosis induced by BLM and candidate GSK-3 as a potential therapeutic target for preventing pulmonary fibrosis.
Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival.[Pubmed:17148450]
J Biol Chem. 2007 Feb 9;282(6):3904-17.
Glycogen synthase kinase-3 (GSK-3) is a serine/threonine kinase consisting of two isoforms, alpha and beta. The activities of GSK-3 are regulated negatively by serine phosphorylation but positively by tyrosine phosphorylation. GSK-3 inactivation has been proposed as a mechanism to promote neuronal survival. We used GSK-3 isoform-specific small interfering RNAs, dominant-negative mutants, or pharmacological inhibitors to search for functions of the two GSK-3 isoforms in regulating neuronal survival in cultured cortical neurons in response to glutamate insult or during neuronal maturation/aging. Surprisingly, RNA interference-induced depletion of either isoform was sufficient to block glutamate-induced excitotoxicity, and the resulting neuroprotection was associated with enhanced N-terminal serine phosphorylation in both GSK-3 isoforms. However, GSK-3beta depletion was more effective than GSK-3alpha depletion in suppressing spontaneous neuronal death in extended culture. This phenomenon is likely due to selective and robust inhibition of GSK-3beta activation resulting from GSK-3beta Ser9 dephosphorylation during the course of spontaneous neuronal death. GSK-3alpha silencing resulted in reduced tyrosine phosphorylation of GSK-3beta, suggesting that tyrosine phosphorylation is also a critical autoregulatory event. Interestingly, GSK-3 inhibitors caused a rapid and long-lasting increase in GSK-3alpha Ser21 phosphorylation levels, followed by a delayed increase in GSK-3beta Ser9 phosphorylation and a decrease in GSK-3alpha Tyr279 and GSK-3beta Tyr216 phosphorylation, thus implying additional levels of GSK-3 autoregulation. Taken together, our results underscore important similarities and dissimilarities of GSK-3alpha and GSK-3beta in the roles of cell survival as well as their distinct modes of regulation. The development of GSK-3 isoform-specific inhibitors seems to be warranted for treating GSK-3-mediated pathology.
Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death.[Pubmed:11279265]
J Neurochem. 2001 Apr;77(1):94-102.
The phosphatidylinositol 3-kinase (PI 3-kinase)/protein kinase B (PKB; also known as Akt) signalling pathway is recognized as playing a central role in the survival of diverse cell types. Glycogen synthase kinase-3 (GSK-3) is a ubiquitously expressed serine/threonine protein kinase that is one of several known substrates of PKB. PKB phosphorylates GSK-3 in response to insulin and growth factors, which inhibits GSK-3 activity and leads to the modulation of multiple GSK-3 regulated cellular processes. We show that the novel potent and selective small-molecule inhibitors of GSK-3; SB-415286 and SB-216763, protect both central and peripheral nervous system neurones in culture from death induced by reduced PI 3-kinase pathway activity. The inhibition of neuronal death mediated by these compounds correlated with inhibition of GSK-3 activity and modulation of GSK-3 substrates tau and beta-catenin. Thus, in addition to the previously assigned roles of GSK-3, our data provide clear pharmacological and biochemical evidence that selective inhibition of the endogenous pool of GSK-3 activity in primary neurones is sufficient to prevent death, implicating GSK-3 as a physiologically relevant principal regulatory target of the PI 3-kinase/PKB neuronal survival pathway.


