1,6-DibromopyreneCAS# 27973-29-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 27973-29-1 | SDF | Download SDF |
| PubChem ID | 176470 | Appearance | Powder |
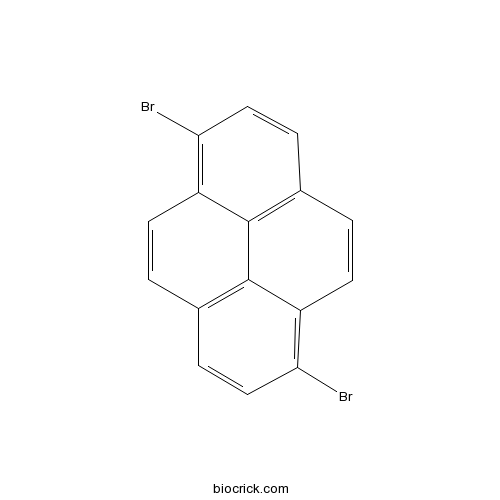
| Formula | C16H8Br2 | M.Wt | 360 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,6-dibromopyrene | ||
| SMILES | C1=CC2=C(C=CC3=C2C4=C1C=CC(=C4C=C3)Br)Br | ||
| Standard InChIKey | JRCJYPMNBNNCFE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H8Br2/c17-13-8-4-10-2-6-12-14(18)7-3-9-1-5-11(13)16(10)15(9)12/h1-8H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1,6-Dibromopyrene Dilution Calculator

1,6-Dibromopyrene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7778 mL | 13.8889 mL | 27.7778 mL | 55.5556 mL | 69.4444 mL |
| 5 mM | 0.5556 mL | 2.7778 mL | 5.5556 mL | 11.1111 mL | 13.8889 mL |
| 10 mM | 0.2778 mL | 1.3889 mL | 2.7778 mL | 5.5556 mL | 6.9444 mL |
| 50 mM | 0.0556 mL | 0.2778 mL | 0.5556 mL | 1.1111 mL | 1.3889 mL |
| 100 mM | 0.0278 mL | 0.1389 mL | 0.2778 mL | 0.5556 mL | 0.6944 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Amino-3-chloro-1,4-naphthoquinone
Catalog No.:BCC8525
CAS No.:2797-51-5
- 14-Deoxy-epsilon-caesalpin
Catalog No.:BCN7254
CAS No.:279683-46-4
- H-Glu(OBzl)-OBzl.TosOH
Catalog No.:BCC2928
CAS No.:2791-84-6
- H-Lys(Z)-OMe.HCl
Catalog No.:BCC2988
CAS No.:27894-50-4
- Bis(4-bromophenyl)acetylene
Catalog No.:BCC8882
CAS No.:2789-89-1
- GW4064
Catalog No.:BCC4500
CAS No.:278779-30-9
- Croceic acid
Catalog No.:BCN2372
CAS No.:27876-94-4
- NH125
Catalog No.:BCC4001
CAS No.:278603-08-0
- Lamiide
Catalog No.:BCN4656
CAS No.:27856-54-8
- Nicergoline
Catalog No.:BCC5214
CAS No.:27848-84-6
- Macrophylline
Catalog No.:BCN1987
CAS No.:27841-97-0
- Loxapine Succinate
Catalog No.:BCC4674
CAS No.:27833-64-3
- Isosteviol
Catalog No.:BCN2685
CAS No.:27975-19-5
- Gardenin B
Catalog No.:BCN3816
CAS No.:2798-20-1
- Bellidifolin
Catalog No.:BCN7424
CAS No.:2798-25-6
- H-Cys(Trt)-OH
Catalog No.:BCC2911
CAS No.:2799-07-7
- Cimigenoside
Catalog No.:BCN5174
CAS No.:27994-11-2
- 25-O-methylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1464
CAS No.:27994-13-4
- 2,6-Bis(2-benzimidazolyl)pyridine
Catalog No.:BCC8504
CAS No.:28020-73-7
- Piperazine Ferulate
Catalog No.:BCN3277
CAS No.:171876-65-6
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
Synthesis of graphene nanoribbons with a defined mixed edge-site sequence by surface assisted polymerization of (1,6)-dibromopyrene on Ag(110).[Pubmed:27714142]
Nanoscale. 2016 Oct 20;8(41):17843-17853.
By a combination of scanning tunneling microscopy, X-ray spectroscopic techniques and density functional theory calculations, we prove the formation of extended patterns of parallel, graphene nanoribbons with alternate zig-zag and armchair edges and selected width by surface-assisted Ullmann coupling polymerization and dehydrogenation of 1,6-Dibromopyrene (C16H8Br2). Besides the relevance of these nanostructures for their possible application in nanodevices, we demonstrate the peculiarity of halogenated pyrene derivatives for the formation of nanoribbons, in particular on Ag(110). These results open the possibility of tuning the shape and dimension of nanoribbons (and hence the correlated electronic properties) by choosing suitably tailored or on-purpose designed molecular precursors.
Emission color tuning and deep blue dopant materials based on 1,6-bis(N-phenyl-p-(R)-phenylamino)pyrene.[Pubmed:19810692]
J Org Chem. 2009 Nov 6;74(21):8472-5.
Panchromatic 1,6-bis(N-phenyl-p-(R)-phenylamino)pyrenes, 2R, were obtained from Buchwald-Hartwig coupling reactions between N-phenyl-p-(R)-phenylamines and 1,6-Dibromopyrene. The photophysical properties of 2R corresponded well to the electron-withdrawing and -donating nature of the diarylamine substituents, exhibiting a full color visible range between 454 and 620 nm. In particular, a deep blue 2CN showed a high radiative rate constant of 2.85 x 10(8) s(-1) with high emission quantum efficiency of 79%. Further applications of 2CN as a blue dopant were attempted using multilayer organic light-emitting devices. A maximum efficiency of 3.98 cd/A with CIE coordinates of x = 0.14, y = 0.10 were obtained.


