H-Cys(Trt)-OHPotent, selective inhibitor of mitotic kinesin Eg5 CAS# 2799-07-7 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2799-07-7 | SDF | Download SDF |
| PubChem ID | 76044 | Appearance | Powder |
| Formula | C22H21NO2S | M.Wt | 363.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | S-Trityl-L-Cysteine;(+)-S-Trityl-L-Cysteine; S-Tritylcysteine; Tritylcysteine | ||
| Solubility | Soluble to 50 mM in DMSO | ||
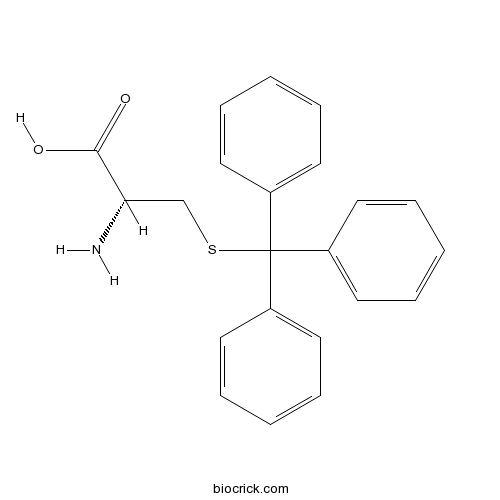
| Chemical Name | (2R)-2-amino-3-tritylsulfanylpropanoic acid | ||
| SMILES | C1=CC=C(C=C1)C(C2=CC=CC=C2)(C3=CC=CC=C3)SCC(C(=O)O)N | ||
| Standard InChIKey | DLMYFMLKORXJPO-FQEVSTJZSA-N | ||
| Standard InChI | InChI=1S/C22H21NO2S/c23-20(21(24)25)16-26-22(17-10-4-1-5-11-17,18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-15,20H,16,23H2,(H,24,25)/t20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, cell-permeable, selective inhibitor of mitotic kinesin Eg5, a protein required for establishing and maintaining a bipolar spindle. Inhibits basal ATPase activity (IC50 = 1 mM) and microtubule-activated ATPase activity of Eg5 (IC50 = 140 nM). Induces mitotic arrest in HeLa cells with an IC50 of 700 nM. Displays antitumor activity. |

H-Cys(Trt)-OH Dilution Calculator

H-Cys(Trt)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.751 mL | 13.7552 mL | 27.5103 mL | 55.0206 mL | 68.7758 mL |
| 5 mM | 0.5502 mL | 2.751 mL | 5.5021 mL | 11.0041 mL | 13.7552 mL |
| 10 mM | 0.2751 mL | 1.3755 mL | 2.751 mL | 5.5021 mL | 6.8776 mL |
| 50 mM | 0.055 mL | 0.2751 mL | 0.5502 mL | 1.1004 mL | 1.3755 mL |
| 100 mM | 0.0275 mL | 0.1376 mL | 0.2751 mL | 0.5502 mL | 0.6878 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Cys(Trt)-OH
- Bellidifolin
Catalog No.:BCN7424
CAS No.:2798-25-6
- Gardenin B
Catalog No.:BCN3816
CAS No.:2798-20-1
- Isosteviol
Catalog No.:BCN2685
CAS No.:27975-19-5
- 1,6-Dibromopyrene
Catalog No.:BCC8428
CAS No.:27973-29-1
- 2-Amino-3-chloro-1,4-naphthoquinone
Catalog No.:BCC8525
CAS No.:2797-51-5
- 14-Deoxy-epsilon-caesalpin
Catalog No.:BCN7254
CAS No.:279683-46-4
- H-Glu(OBzl)-OBzl.TosOH
Catalog No.:BCC2928
CAS No.:2791-84-6
- H-Lys(Z)-OMe.HCl
Catalog No.:BCC2988
CAS No.:27894-50-4
- Bis(4-bromophenyl)acetylene
Catalog No.:BCC8882
CAS No.:2789-89-1
- GW4064
Catalog No.:BCC4500
CAS No.:278779-30-9
- Croceic acid
Catalog No.:BCN2372
CAS No.:27876-94-4
- NH125
Catalog No.:BCC4001
CAS No.:278603-08-0
- Cimigenoside
Catalog No.:BCN5174
CAS No.:27994-11-2
- 25-O-methylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1464
CAS No.:27994-13-4
- 2,6-Bis(2-benzimidazolyl)pyridine
Catalog No.:BCC8504
CAS No.:28020-73-7
- Piperazine Ferulate
Catalog No.:BCN3277
CAS No.:171876-65-6
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- (+)-Ulopterol
Catalog No.:BCN1228
CAS No.:28095-18-3
- Chaetocin
Catalog No.:BCC2429
CAS No.:28097-03-2
- Adamantane
Catalog No.:BCN8481
CAS No.:281-23-2
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
Preparation of protected peptidyl thioester intermediates for native chemical ligation by Nalpha-9-fluorenylmethoxycarbonyl (Fmoc) chemistry: considerations of side-chain and backbone anchoring strategies, and compatible protection for N-terminal cysteine.[Pubmed:15787970]
J Pept Res. 2005 Mar;65(3):395-410.
Native chemical ligation has proven to be a powerful method for the synthesis of small proteins and the semisynthesis of larger ones. The essential synthetic intermediates, which are C-terminal peptide thioesters, cannot survive the repetitive piperidine deprotection steps of N(alpha)-9-fluorenylmethoxycarbonyl (Fmoc) chemistry. Therefore, peptide scientists who prefer to not use N(alpha)-t-butyloxycarbonyl (Boc) chemistry need to adopt more esoteric strategies and tactics in order to integrate ligation approaches with Fmoc chemistry. In the present work, side-chain and backbone anchoring strategies have been used to prepare the required suitably (partially) protected and/or activated peptide intermediates spanning the length of bovine pancreatic trypsin inhibitor (BPTI). Three separate strategies for managing the critical N-terminal cysteine residue have been developed: (i) incorporation of N(alpha)-9-fluorenylmethoxycarbonyl-S-(N-methyl-N-phenylcarbamoyl)sulfenylcystein e [Fmoc-Cys(Snm)-OH], allowing creation of an otherwise fully protected resin-bound intermediate with N-terminal free Cys; (ii) incorporation of N(alpha)-9-fluorenylmethoxycarbonyl-S-triphenylmethylcysteine [Fmoc-Cys(Trt)-OH], generating a stable Fmoc-Cys(H)-peptide upon acidolytic cleavage; and (iii) incorporation of N(alpha)-t-butyloxycarbonyl-S-fluorenylmethylcysteine [Boc-Cys(Fm)-OH], generating a stable H-Cys(Fm)-peptide upon cleavage. In separate stages of these strategies, thioesters are established at the C-termini by selective deprotection and coupling steps carried out while peptides remain bound to the supports. Pilot native chemical ligations were pursued directly on-resin, as well as in solution after cleavage/purification.
[Synthesis of the fragments A1-8, A1-7, A9-15 and A8-15 of the chicken insulin A chain (author's transl)].[Pubmed:511092]
Hoppe Seylers Z Physiol Chem. 1979 Nov;360(11):1559-68.
By coupling the peptide derivatives H-Cys(SBut)-Cys(SBut)-His-OMe(6-8 b) and H-Cys(SBut)-Cys(SBut)-OH(6-7b) respectively with Trt-Gly-Ile-Val-Glu(OBut)-Gln-OH(1-5a) the N-terminal sequences A1-8 and A1-7 of the chicken insulin A chain have been prepared. The sequence of A9-15 has been obtained by connecting Bpoc-Asn-Thr(But)-Cys(SBut)-OH (9-11c) and H-Ser(But)-Leu-Try(But)-Gln-OH (12-15). Acylation of the aminopeptidderivative 9-15b with Bpoc-N2H3 yielded fragment A8-15 (8-15).
Lipoconjugates: structure-activity studies for pheromone analogues of Ustilago maydis with varied lipophilicity.[Pubmed:8919059]
Int J Pept Protein Res. 1996 Oct;48(4):377-90.
The synthesis, biological activities and conformational behaviour of a variety of analogues of the mating pheromones of the basidomycete Ustilago maydis are reported. The pheromone analogues derived from the two allelic forms H-G-R-D-N-G-S-P-I-G-Y-S-S-Xaa-Z (a1) and H-N-R-G-Q-P-G-Y-Y-Xaa-Z (a2), with Xaa-Z being an unidentified lipophilic cysteine derivative, all differ in the C-terminal residue and include -Cys(farnesyl)-OMe, -Cys(farnesyl)-OH, -Cys(prenyl)-OMe, -Cys-OMe, -Cys(n-dodecyl)-OMe and the unnatural residues -Ahds-OMe (Ahds=alpha-aminohexadecanoic acid), -Ahds-OH, -Ads-OMe (Ads=alpha-aminodecanoic acid) and -N-Hdg-OMe (N-Hdg=N-hexadecylglycine). The synthesis of the unnatural methyl ester analogues was carried out by condensation of the fully protected fragments Fmoc-G-R(Pmc)-D(tBu)-N(Trt)-G-S(tBu)-P-I-G-Y(tBu)-S(tBu)-S(tBu)-OH (a1') and Fmoc-N(Trt)-R(Pmc)-G-Q(Trt)-P-G-Y(tBu)-Y(tBu)-OH (a2') respectively, prepared by Fmoc-SPPS, with the appropriate methylester compounds and subsequent deprotection with TFA/scavenger and piperidine. Synthesis and physicochemical properties of the unnatural lipophilic amino acid methylesters are described. The preparation of the cysteine analogues was performed by condensation of a1' or a2' with H-Cys(Trt)-OMe and subsequent deprotection with TFA/scavenger. Alkylation of the thiol function and Fmoc-deprotection was achieved in a novel one-pot reaction by treatment with alkyl bromide and DIPEA, quenching with EDT and Fmoc removal by addition of 20% piperidine (v/v). Hydrolysis of the methyl esters was carried out by treatment with NaOH in MeOH/H2O. The results of the biological assay reveal an increase in activity with increasing chain length of the lipophilic anchor, with alkyl being better than prenyl and sulfur being not essential, while the position of the anchor is optimal at C7 and the methyl ester moiety is important. NMR studies of two chosen analogues in DMSO and SDS/water demonstrate that the lipophilic C-terminal residue has no influence on the structural behaviour of the peptides. Chemical-shift and NOE patterns indicate a main all-trans conformation of the peptide backbone and a weakly populated cis conformation around the Xaa Pro peptide bond in all eight cases without formation of a defined folded structure. No evidence is seen that the membrane-simulating system SDS/water has a structure-inducing effect on the bound peptide. We therefore conclude that the lipomodification in mating pheromones of U. maydis acts to increase the effective concentration of the drug in the target cell membrane without additional structure-inducing or receptor-binding effects.
Synthesis and acid ionization constants of cyclic cystine peptides H-Cys-(Gly)n-Cys-OH (n = 0-4).[Pubmed:2599775]
Int J Pept Protein Res. 1989 Oct;34(4):346-51.
Cyclic peptide disulfides of the general formula H-Cys-(Gly)n-Cys-OH (n = 0-4) were synthesized from the corresponding peptide derivatives [Boc-Cys(Trt)(Gly)-n-Cys(Trt)-OBut] by oxidation with iodine in methanol and by subsequent removal of the terminal groups with trifluoroacetic acid. Acid ionization constants of the obtained peptides were determined by potentiometric titration in aqueous KCl (0.1 mol/L) medium. All compounds have two dissociable hydrogens, corresponding to carboxyl (pK1 = 2.35-2.84) and to terminal amino group (pK2 = 5.61-6.93); pK1 values show first an upward and then a downward trend with the increase in ring size; the opposite is true for pK2 values. These trends could be tentatively attributed to the intramolecular salt bridge (-COO- ----NH+3-) formation.
Identification of the protein binding region of S-trityl-L-cysteine, a new potent inhibitor of the mitotic kinesin Eg5.[Pubmed:15476401]
Biochemistry. 2004 Oct 19;43(41):13072-82.
Human Eg5, a mitotic motor of the kinesin superfamily, is involved in the formation and maintenance of the mitotic spindle. The recent discovery of small molecules that inhibit HsEg5 by binding to its catalytic motor domain leading to mitotic arrest has attracted more interest in Eg5 as a potential anticancer drug target. We have used hydrogen-deuterium exchange mass spectrometry and directed mutagenesis to identify the secondary structure elements that form the binding sites of new Eg5 inhibitors, in particular for S-trityl-l-cysteine, a potent inhibitor of Eg5 activity in vitro and in cell-based assays. The binding of this inhibitor modifies the deuterium incorporation rate of eight peptides that define two areas within the motor domain: Tyr125-Glu145 and Ile202-Leu227. Replacement of the Tyr125-Glu145 region with the equivalent region in the Neurospora crassa conventional kinesin heavy chain prevents the inhibition of the Eg5 ATPase activity by S-trityl-l-cysteine. We show here that S-trityl-l-cysteine and monastrol both bind to the same region on Eg5 by induced fit in a pocket formed by helix alpha3-strand beta5 and loop L5-helix alpha2, and both inhibitors trigger similar local conformational changes within the interaction site. It is likely that S-trityl-l-cysteine and monastrol inhibit HsEg5 by a similar mechanism. The common inhibitor binding region appears to represent a "hot spot" for HsEg5 that could be exploited for further inhibitor screening.
In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities.[Pubmed:15367702]
Mol Cancer Ther. 2004 Sep;3(9):1079-90.
Human Eg5, a member of the kinesin superfamily, plays a key role in mitosis, as it is required for the formation of a bipolar spindle. We describe here the first in vitro microtubule-activated ATPase-based assay for the identification of small-molecule inhibitors of Eg5. We screened preselected libraries obtained from the National Cancer Institute and identified S-trityl-L-cysteine as the most effective Eg5 inhibitor with an IC50 of 1.0 micromol/L for the inhibition of basal ATPase activity and 140 nmol/L for the microtubule-activated ATPase activity. Subsequent cell-based assays revealed that S-trityl-L-cysteine induced mitotic arrest in HeLa cells (IC50, 700 nmol/L) with characteristic monoastral spindles. S-trityl-L-cysteine is 36 times more potent for inducing mitotic arrest than the well-studied inhibitor, monastrol. Gossypol, flexeril, and two phenothiazine analogues were also identified as Eg5 inhibitors, and we found that they all result in monoastral spindles in HeLa cells. It is notable that all the Eg5 inhibitors identified here have been shown previously to inhibit tumor cell line growth in the NCI 60 tumor cell line screen, and we conclude that their antitumor activity may at least in part be explained by their ability to inhibit Eg5 activity.


