(+)-UlopterolCAS# 28095-18-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28095-18-3 | SDF | Download SDF |
| PubChem ID | 176475 | Appearance | Powder |
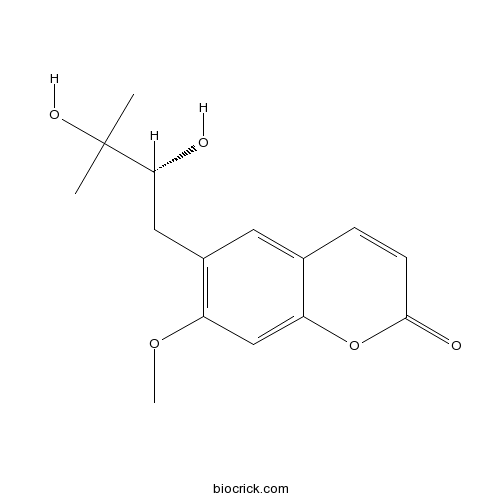
| Formula | C15H18O5 | M.Wt | 278.30 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 36149-96-9 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-[(2R)-2,3-dihydroxy-3-methylbutyl]-7-methoxychromen-2-one | ||
| SMILES | CC(C)(C(CC1=C(C=C2C(=C1)C=CC(=O)O2)OC)O)O | ||
| Standard InChIKey | BNLKKFPQJANWMM-CYBMUJFWSA-N | ||
| Standard InChI | InChI=1S/C15H18O5/c1-15(2,18)13(16)7-10-6-9-4-5-14(17)20-12(9)8-11(10)19-3/h4-6,8,13,16,18H,7H2,1-3H3/t13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (R)-peucedanol has radical scavenging on 1,1-diphenyl-2-picrylhydrazyl radical and for inhibition of oxidation of liposome induced by 2,2'-azobis(2-amidinopropane) dihydrochloride, it may have antioxidant activity. |

(+)-Ulopterol Dilution Calculator

(+)-Ulopterol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5932 mL | 17.9662 mL | 35.9324 mL | 71.8649 mL | 89.8311 mL |
| 5 mM | 0.7186 mL | 3.5932 mL | 7.1865 mL | 14.373 mL | 17.9662 mL |
| 10 mM | 0.3593 mL | 1.7966 mL | 3.5932 mL | 7.1865 mL | 8.9831 mL |
| 50 mM | 0.0719 mL | 0.3593 mL | 0.7186 mL | 1.4373 mL | 1.7966 mL |
| 100 mM | 0.0359 mL | 0.1797 mL | 0.3593 mL | 0.7186 mL | 0.8983 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- Piperazine Ferulate
Catalog No.:BCN3277
CAS No.:171876-65-6
- 2,6-Bis(2-benzimidazolyl)pyridine
Catalog No.:BCC8504
CAS No.:28020-73-7
- 25-O-methylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1464
CAS No.:27994-13-4
- Cimigenoside
Catalog No.:BCN5174
CAS No.:27994-11-2
- H-Cys(Trt)-OH
Catalog No.:BCC2911
CAS No.:2799-07-7
- Bellidifolin
Catalog No.:BCN7424
CAS No.:2798-25-6
- Gardenin B
Catalog No.:BCN3816
CAS No.:2798-20-1
- Isosteviol
Catalog No.:BCN2685
CAS No.:27975-19-5
- Chaetocin
Catalog No.:BCC2429
CAS No.:28097-03-2
- Adamantane
Catalog No.:BCN8481
CAS No.:281-23-2
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
Novel coumarin and furan from the roots of Angelica pubescens f. biserrata.[Pubmed:20183310]
J Asian Nat Prod Res. 2009 Aug;11(8):698-703.
A new natural coumarin, angepubebisin (1), and a new furan, angepubefurin (2), together with the five known compounds, umbelliferone, angelol B (3), ulopterol (4), peucedanol (5), and scopoletin, were isolated from the roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan. The structures of angepubebisin (1) and known compounds were determined by spectroscopic methods, including IR, UV, EI-MS, HR-FTICR-MS, 1D-, and 2D-NMR spectral analyses, and angepubefurin (2) was determined by HR-FTICR-MS and X-ray diffraction analyses.
Transport of Twelve Coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR Cell Monolayer-An in Vitro Model for Blood-Brain Barrier Permeability.[Pubmed:26121397]
Molecules. 2015 Jun 25;20(7):11719-32.
Angelicae Pubescentis Radix (APR), a widely used traditional Chinese medicine, is reported to have central nervous system activities. The purpose of this study was to characterize the blood-brain barrier permeability of twelve coumarins from APR including umbelliferone (1), osthol (2), scopoletin (3), peucedanol (4), ulopterol (5), angepubebisin (6), psoralen (7), xanthotoxin (8), bergapten (9), isoimperatorin (10), columbianadin (11), and columbianetin acetate (12) with an in vitro model using a MDCK-pHaMDR cell monolayer. The cell monolayer was validated to be suitable for the permeation experiments. The samples' transports were analyzed by high performance liquid chromatography and their apparent permeability coefficients (Papp) were calculated. According to the Papp value, most coumarins could be characterized as well-absorbed compounds except for 4, 10 and 11 which were moderately absorbed ones, in concentration-dependent and time-dependent manners. The results of P-glycoprotein (P-gp) inhibitor (verapamil) experiments showed that the transport of coumarin 4 was affected by the transport protein P-gp. Sigmoid functions between permeability log(Papp AP-BL*MW0.5) and log D (at pH 7.4) were established to analyze the structure-activity relationship of coumarins. The results provide useful information for discovering the substance basis for the central nervous system activities of APR, and predicting the permeability of other coumarins through BBB.
Antioxidant compounds from the leaves of Peucedanum japonicum thunb.[Pubmed:12926867]
J Agric Food Chem. 2003 Aug 27;51(18):5255-61.
Seventeen compounds were isolated from the n-butanol soluble fraction of the leaves of Peucedanum japonicum Thunb. On the basis of MS and various NMR spectroscopic techniques, the structures of the isolated compounds were determined as isoquercitrin (1), rutin (2), 3-O-caffeoylquinic acid (3), 4-O-caffeoylquinic acid (4), 5-O-caffeoylquinic acid (5), cnidioside A (6), praeroside II (7), praeroside III (8), apterin (9), esculin (10), (R)-peucedanol (11), (R)-peucedanol 7-O-beta-d-glucopyranoside (12), l-tryptophan (13), uracil (14), guanosine (15), uridine (16), and thymidine (17). All compounds except 11 and 12 were isolated for the first time from P. japonicum. Several isolated compounds were quantified by high-performance liquid chromatography analysis. In addition, all isolated compounds were examined for radical scavenging on 1,1-diphenyl-2-picrylhydrazyl radical and for inhibition of oxidation of liposome induced by 2,2'-azobis(2-amidinopropane)dihydrochloride. Compounds 2-5 were found to be the major potent constituents, which contribute to the antioxidant activity of P. japonicum leaves.


