AdamantaneCAS# 281-23-2 |
Quality Control & MSDS
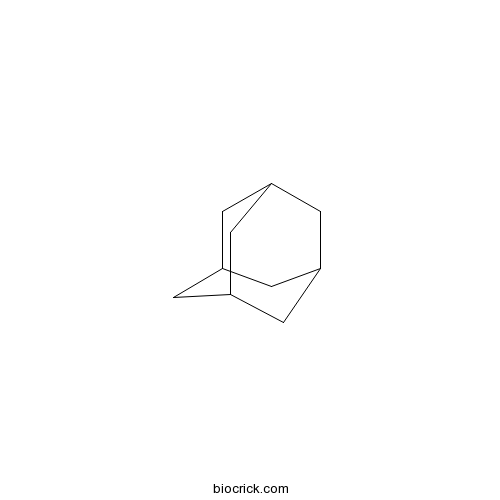
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 281-23-2 | SDF | Download SDF |
| PubChem ID | 9238 | Appearance | Colorless crystal |
| Formula | C10H16 | M.Wt | 136.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | adamantane | ||
| SMILES | C1C2CC3CC1CC(C2)C3 | ||
| Standard InChIKey | ORILYTVJVMAKLC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H16/c1-7-2-9-4-8(1)5-10(3-7)6-9/h7-10H,1-6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Adamantane Dilution Calculator

Adamantane Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.3405 mL | 36.7026 mL | 73.4053 mL | 146.8105 mL | 183.5132 mL |
| 5 mM | 1.4681 mL | 7.3405 mL | 14.6811 mL | 29.3621 mL | 36.7026 mL |
| 10 mM | 0.7341 mL | 3.6703 mL | 7.3405 mL | 14.6811 mL | 18.3513 mL |
| 50 mM | 0.1468 mL | 0.7341 mL | 1.4681 mL | 2.9362 mL | 3.6703 mL |
| 100 mM | 0.0734 mL | 0.367 mL | 0.7341 mL | 1.4681 mL | 1.8351 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chaetocin
Catalog No.:BCC2429
CAS No.:28097-03-2
- (+)-Ulopterol
Catalog No.:BCN1228
CAS No.:28095-18-3
- A 286982
Catalog No.:BCC3946
CAS No.:280749-17-9
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- VUF 5574
Catalog No.:BCC7030
CAS No.:280570-45-8
- Rediocide A
Catalog No.:BCN5175
CAS No.:280565-85-7
- Piperazine Ferulate
Catalog No.:BCN3277
CAS No.:171876-65-6
- 2,6-Bis(2-benzimidazolyl)pyridine
Catalog No.:BCC8504
CAS No.:28020-73-7
- 25-O-methylcimigenol-3-O-beta-D-xylopyranoside
Catalog No.:BCN1464
CAS No.:27994-13-4
- Cimigenoside
Catalog No.:BCN5174
CAS No.:27994-11-2
- H-Cys(Trt)-OH
Catalog No.:BCC2911
CAS No.:2799-07-7
- Bellidifolin
Catalog No.:BCN7424
CAS No.:2798-25-6
- Pluviatolide
Catalog No.:BCN3041
CAS No.:28115-68-6
- H-Pro-OtBu
Catalog No.:BCC3020
CAS No.:2812-46-6
- 8-Aminoadenine
Catalog No.:BCC6108
CAS No.:28128-33-8
- Peonidin-3-O-galactoside chloride
Catalog No.:BCN3027
CAS No.:28148-89-2
- CHC
Catalog No.:BCC7994
CAS No.:28166-41-8
- Futoquinol
Catalog No.:BCN6416
CAS No.:28178-92-9
- 5-Amino-2-mercaptobenzimidazole
Catalog No.:BCC8730
CAS No.:2818-66-8
- Sinensin
Catalog No.:BCN4797
CAS No.:28189-90-4
- Dihydrodehydrodiconiferyl alcohol
Catalog No.:BCN5176
CAS No.:28199-69-1
- Lauterine
Catalog No.:BCN7062
CAS No.:28200-65-9
- JTE 907
Catalog No.:BCC7380
CAS No.:282089-49-0
- Phlorin
Catalog No.:BCN5177
CAS No.:28217-60-9
Novel chelators based on adamantane-derived semicarbazones and hydrazones that target multiple hallmarks of Alzheimer's disease.[Pubmed:29749416]
Dalton Trans. 2018 May 29;47(21):7190-7205.
Alzheimer's disease (AD) is characterized by multiple pathological hallmarks, including beta-amyloid aggregation, oxidative stress, and metal dys-homeostasis. In the absence of treatments addressing its multi-factorial pathology, we designed novel multi-functional Adamantane-based semicarbazones and hydrazones (1-12) targeting AD hallmarks. Of these, 2-pyridinecarboxaldehyde (N-adamantan-1-yl)benzoyl-4-amidohydrazone (10) was identified as the lead compound, which demonstrated: (1) pronounced iron chelation efficacy; (2) attenuation of CuII-mediated beta-amyloid aggregation; (3) low cytotoxicity; (4) inhibition of oxidative stress; and (5) favorable characteristics for effective blood-brain barrier permeation. Structure-activity relationships revealed that pyridine-derived hydrazones represent a promising pharmacophore for future design strategies due to their ability to bind critical FeII pools. Collectively, the unique multi-functional activity of these agents provides a novel therapeutic strategy for AD treatment.
Novel Guanidine Compound against Multidrug-Resistant Cystic Fibrosis-Associated Bacterial Species.[Pubmed:29751676]
Molecules. 2018 May 11;23(5). pii: molecules23051158.
Chronic pulmonary infection is a hallmark of lung disease in cystic fibrosis (CF). Infections dominated by non-fermentative Gram-negative bacilli are particularly difficult to treat and highlight an urgent need for the development of new class of agents to combat these infections. In this work, a small library comprising thiourea and guanidine derivatives with low molecular weight was designed; these derivatives were studied as antimicrobial agents against Gram-positive, Gram-negative, and a panel of drug-resistant clinical isolates recovered from patients with CF. One novel compound, a guanidine derivative bearing Adamantane-1-carbonyl and 2-bromo-4,6-difluouro-phenyl substituents (H-BDF), showed potent bactericidal activity against the strains tested, at levels generally higher than those exhibited by tobramycin, ceftazimide and meropenem. The role that different substituents exert in the antimicrobial activity has been determined, highlighting the importance of the halo-phenyl group in the guanidine moiety. The new compound displays low levels of cytotoxicity against THP-1 and A549 cells with a selective index (SI) > 8 (patent application PCT/IB2017/054870, August 2017). Taken together, our results indicate that H-BDF can be considered as a promising antimicrobial agent.
Assembling of stimuli-responsive tumor targeting polypyrrole nanotubes drug carrier system for controlled release.[Pubmed:29752103]
Mater Sci Eng C Mater Biol Appl. 2018 Aug 1;89:316-327.
A stimuli-responsive polypyrrole (PPy) nanotubes drug carrier system has been designed to deliver anticancer drugs to tumor cells in a targeted and controlled manner. The PPy nanotubes drug carrier was fabricated by a template method. The nanotubes surface was functionalized with cleavable acylhydrazone and disulfide bonds by attaching thiolated beta-cyclodextrin (beta-CD). The solubilizing poly(ethylene glycol) polymer (PEG), attached with an Adamantane (Ad) entity at one end and a folate (FA) entity at the other end, was introduced onto the nanotubes surface via beta-cyclodextrin-Adamantane interaction. The synthesized FA-PEG-Ad-beta-CD-PPy showed excellent biocompatibility and low cytotoxicity for two cell lines. Doxorubicin (Dox) loaded FA-PEG-Ad-beta-CD-PPy nanotubes showed a triggered in vitro drug release behavior in the presence of acidic media and reducing agents. The folate-mediated endocytosis and intracellular release of Dox-loaded nanoparticles were confirmed by fluorescence microscopy and cell viability evaluations. In the in vitro study, Dox loaded within the nanoparticles showed enhanced selectivity for cancerous cells and reduced cytotoxicity for normal cells compared to free Dox. The PPy based targeted drug vehicle shows excellent promise for drug delivery.
A Supramolecular Approach for Liver Radioembolization.[Pubmed:29721086]
Theranostics. 2018 Mar 23;8(9):2377-2386.
Hepatic radioembolization therapies can suffer from discrepancies between diagnostic planning (scout-scan) and the therapeutic delivery itself, resulting in unwanted side-effects such as pulmonary shunting. We reasoned that a nanotechnology-based pre-targeting strategy could help overcome this shortcoming by directly linking pre-interventional diagnostics to the local delivery of therapy. Methods: The host-guest interaction between Adamantane and cyclodextrin was employed in an in vivo pre-targeting set-up. Adamantane (guest)-functionalized macro albumin aggregates (MAA-Ad; d = 18 mum) and (radiolabeled) Cy5 and beta-cyclodextrin (host)-containing PIBMA polymers ((99m)Tc-Cy50.5CD10PIBMA39; MW ~ 18.8 kDa) functioned as the reactive pair. Following liver or lung embolization with ((99m)Tc)-MAA-Ad or ((99m)Tc)-MAA (control), the utility of the pre-targeting concept was evaluated after intravenous administration of (99m)Tc-Cy50.5CD10PIBMA39. Results: Interactions between MAA-Ad and Cy50.5CD10PIBMA39 could be monitored in solution using confocal microscopy and were quantified by radioisotope-based binding experiments. In vivo the accumulation of the MAA-Ad particles in the liver or lungs yielded an approximate ten-fold increase in accumulation of (99m)Tc-Cy50.5CD10PIBMA39 in these organs (16.2 %ID/g and 10.5 %ID/g, respectively) compared to the control. Pre-targeting with MAA alone was shown to be only half as efficient. Uniquely, for the first time, this data demonstrates that the formation of supramolecular interactions between cyclodextrin and Adamantane can be used to drive complex formation in the chemically challenging in vivo environment. Conclusion: The in vivo distribution pattern of the cyclodextrin host could be guided by the pre-administration of the Adamantane guest, thereby creating a direct link between the scout-scan (MAA-Ad) and delivery of therapy.
KO(t)Bu as a Single Electron Donor? Revisiting the Halogenation of Alkanes with CBr(4) and CCl(4).[Pubmed:29724009]
Molecules. 2018 May 1;23(5). pii: molecules23051055.
The search for reactions where KO(t)Bu and other tert-alkoxides might behave as single electron donors led us to explore their reactions with tetrahalomethanes, CX(4), in the presence of Adamantane. We recently reported the halogenation of Adamantane under these conditions. These reactions appeared to mirror the analogous known reaction of NaOH with CBr(4) under phase-transfer conditions, where initiation features single electron transfer from a hydroxide ion to CBr(4). We now report evidence from experimental and computational studies that KO(t)Bu and other alkoxide reagents do not go through an analogous electron transfer. Rather, the alkoxides form hypohalites upon reacting with CBr(4) or CCl(4), and homolytic decomposition of appropriate hypohalites initiates the halogenation of Adamantane.
Charge Determines Guest Orientation: A Combined NMR and Molecular Dynamics Study of beta-Cyclodextrins and Adamantane Derivatives.[Pubmed:29688729]
J Phys Chem B. 2018 May 10;122(18):4821-4827.
The strong binding of the adamantyl moiety to the cavity of beta-cyclodextrin makes it a common binding motif in supramolecular chemistry and a common model system. Despite the attention, there are still unresolved questions regarding the orientation of the Adamantane derivatives in the inclusion complexes-do they protrude from the wide or narrow opening of the cyclodextrin hosts? A combined analysis of ROESY NMR and molecular dynamics simulations allows the conclusion that positively charged Adamantane derivatives are oriented with the hydrophilic group protruding from the wider opening of the cyclodextrin, while negatively charged Adamantane derivatives form two coexisting types of complexes where the hydrophilic group respectively protrudes from the wide and narrow opening. Interestingly, structural modifications of the cyclodextrin host only have a slight impact on the guest orientation.
Symmetric dimeric adamantanes for exploring the structure of two viroporins: influenza virus M2 and hepatitis C virus p7.[Pubmed:29750015]
Drug Des Devel Ther. 2018 Apr 30;12:1019-1031.
Background: Adamantane-based compounds have been identified to interfere with the ion-channel activity of viroporins and thereby inhibit viral infection. To better understand the difference in the inhibition mechanism of viroporins, we synthesized symmetric dimeric Adamantane analogs of various alkyl-spacer lengths. Methods: Symmetric dimeric Adamantane derivatives were synthesized where two amantadine or rimantadine molecules were linked by various alkyl-spacers. The inhibitory activity of the compounds was studied on two viroporins: the influenza virus M2 protein, expressed in Xenopus oocytes, using the two-electrode voltage-clamp technique, and the hepatitis C virus (HCV) p7 channels for five different genotypes (1a, 1b, 2a, 3a, and 4a) expressed in HEK293 cells using whole-cell patch-clamp recording techniques. Results: Upon testing on M2 protein, dimeric compounds showed significantly lower inhibitory activity relative to the monomeric amantadine. The lack of channel blockage of the dimeric amantadine and rimantadine analogs against M2 wild type and M2-S31N mutant was consistent with previously proposed drug-binding mechanisms and further confirmed that the pore-binding model is the pharmacologically relevant drug-binding model. On the other hand, these dimers showed similar potency to their respective monomeric analogs when tested on p7 protein in HCV genotypes 1a, 1b, and 4a while being 700-fold and 150-fold more potent than amantadine in genotypes 2a and 3a, respectively. An amino group appears to be important for inhibiting the ion-channel activity of p7 protein in genotype 2a, while its importance was minimal in all other genotypes. Conclusion: Symmetric dimeric Adamantanes can be considered a prospective class of p7 inhibitors that are able to address the differences in Adamantane sensitivity among the various genotypes of HCV.


