chroman 1ROCK II inhibitor, highly potent and selective CAS# 1273579-40-0 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1273579-40-0 | SDF | Download SDF |
| PubChem ID | 66577033 | Appearance | Powder |
| Formula | C24H28N4O4 | M.Wt | 436.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ROCK-II inhibitor | ||
| Solubility | DMSO : ≥ 50 mg/mL (114.55 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
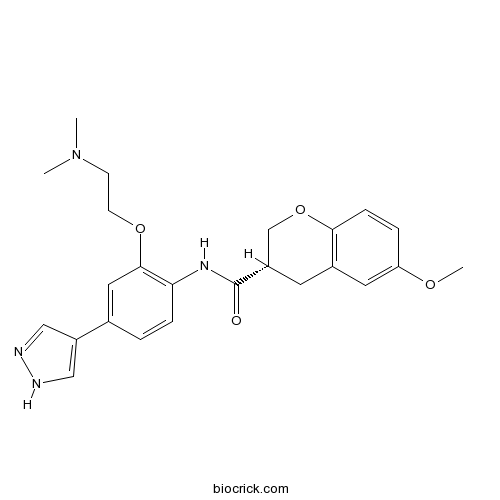
| Chemical Name | (3S)-N-[2-[2-(dimethylamino)ethoxy]-4-(1H-pyrazol-4-yl)phenyl]-6-methoxy-3,4-dihydro-2H-chromene-3-carboxamide | ||
| SMILES | CN(C)CCOC1=C(C=CC(=C1)C2=CNN=C2)NC(=O)C3CC4=C(C=CC(=C4)OC)OC3 | ||
| Standard InChIKey | ROFMCPHQNWGXGE-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C24H28N4O4/c1-28(2)8-9-31-23-12-16(19-13-25-26-14-19)4-6-21(23)27-24(29)18-10-17-11-20(30-3)5-7-22(17)32-15-18/h4-7,11-14,18H,8-10,15H2,1-3H3,(H,25,26)(H,27,29)/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Chroman 1 is a highly potent ROCK2 inhibitor, with an IC50 of < 1 nM.In Vitro:Chroman 1 is a highly potent ROCK2 inhibitor, with an IC50 of < 1 nM. Chroman 1 also shows inhibitory activities against MRCK, with an IC50 of 150 nM, but has no effect on PKA (IC50, > 20000 nM) or AKT1 (IC50, > 20000 nM)[1]. References: | |||||

chroman 1 Dilution Calculator

chroman 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.291 mL | 11.4548 mL | 22.9095 mL | 45.819 mL | 57.2738 mL |
| 5 mM | 0.4582 mL | 2.291 mL | 4.5819 mL | 9.1638 mL | 11.4548 mL |
| 10 mM | 0.2291 mL | 1.1455 mL | 2.291 mL | 4.5819 mL | 5.7274 mL |
| 50 mM | 0.0458 mL | 0.2291 mL | 0.4582 mL | 0.9164 mL | 1.1455 mL |
| 100 mM | 0.0229 mL | 0.1145 mL | 0.2291 mL | 0.4582 mL | 0.5727 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: <1 nM for ROCK-II
Chroman 1 is a highly potent ROCK-II inhibitor. ROCK is a member of the AGC kinase family of serine/threonine kinases that is comprised of two highly homologous isoforms, which are ROCK-I and ROCK-II.. As one of the downstream effectors of RhoA, ROCK regulates stress fiber formation and actin cytoskeletal organization via phosphorylation of myosin light chain. Currently, there is plenty of interest in the inhibition of Rho kinase (ROCK) to treat various diseases.
In vitro: Chroman 1 was found to be a sub-nanomolar ROCK-II inhibitor with excellent to moderate selectivity over related kinases studied as a preliminary assessment, such as AKT1, protein kinase A (PKA), and the highly homologous Cdc42-binding kinase. In addition, Chroman 1 also showed good in-vitro activity in the functional cell-based myosin light chain bis-phosphorylation assay [1].
In vivo: Pharmacokinetic evaluation in rats showed that Chroman 1 had moderately improved oral bioavailability (27–35%) relative to its lead compounds, which were benzodioxane and chroman. In addition, Chroman 1 also displayed an unique intriguing pharmacokinetic profile, which was with high systemic exposure through oral delivery [1].
Clinical trial: So far, there is no clinical study reported.
References:
[1] Chen YT,Bannister TD,Weiser A,Griffin E,Lin L,Ruiz C,Cameron MD,Schürer S,Duckett D,Schrter T,LoGrasso P,Feng Y. Chroman-3-amides as potent Rho kinase inhibitors. Bioorg Med Chem Lett.2008 Dec 15;18(24):6406-9.
- Coclauril
Catalog No.:BCN6150
CAS No.:127350-68-9
- Rebaudioside G
Catalog No.:BCN7860
CAS No.:127345-21-5
- 2-Chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine hydrochloride
Catalog No.:BCC8569
CAS No.:127337-60-4
- YLF-466D
Catalog No.:BCC4086
CAS No.:1273323-67-3
- PACAP 1-27
Catalog No.:BCC5726
CAS No.:127317-03-7
- Zamifenacin fumarate
Catalog No.:BCC7418
CAS No.:127308-98-9
- BRL 37344, sodium salt
Catalog No.:BCC6860
CAS No.:127299-93-8
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Sitafloxacin
Catalog No.:BCC5164
CAS No.:127254-12-0
- Intermedin B
Catalog No.:BCN7317
CAS No.:127214-87-3
- Bisacurone C
Catalog No.:BCN7316
CAS No.:127214-86-2
- (3S,4S)-3-(Boc-amino)-4-methylpyrrolidine
Catalog No.:BCC4015
CAS No.:127199-54-6
- Odoroside A
Catalog No.:BCC8224
CAS No.:12738-19-1
- Y-27152
Catalog No.:BCC7254
CAS No.:127408-30-4
- Y-26763
Catalog No.:BCC7253
CAS No.:127408-31-5
- Calystegine B2
Catalog No.:BCN1879
CAS No.:127414-85-1
- Calystegine B1
Catalog No.:BCN1882
CAS No.:127414-86-2
- Conantokin-T
Catalog No.:BCC5977
CAS No.:127476-26-0
- VU 0360223
Catalog No.:BCC6159
CAS No.:1274859-33-4
- CNX1351
Catalog No.:BCC6375
CAS No.:1276105-89-5
- HS-173
Catalog No.:BCC5363
CAS No.:1276110-06-5
- PF-3644022
Catalog No.:BCC6136
CAS No.:1276121-88-0
- Fananserin
Catalog No.:BCC7440
CAS No.:127625-29-0
- Oleficin
Catalog No.:BCN1848
CAS No.:12764-54-4
Chroman-4-One Derivatives Targeting Pteridine Reductase 1 and Showing Anti-Parasitic Activity.[Pubmed:28282886]
Molecules. 2017 Mar 8;22(3). pii: molecules22030426.
Flavonoids have previously been identified as antiparasitic agents and pteridine reductase 1 (PTR1) inhibitors. Herein, we focus our attention on the chroman-4-one scaffold. Three chroman-4-one analogues (1-3) of previously published chromen-4-one derivatives were synthesized and biologically evaluated against parasitic enzymes (Trypanosoma brucei PTR1-TbPTR1 and Leishmania major-LmPTR1) and parasites (Trypanosoma brucei and Leishmania infantum). A crystal structure of TbPTR1 in complex with compound 1 and the first crystal structures of LmPTR1-flavanone complexes (compounds 1 and 3) were solved. The inhibitory activity of the chroman-4-one and chromen-4-one derivatives was explained by comparison of observed and predicted binding modes of the compounds. Compound 1 showed activity both against the targeted enzymes and the parasites with a selectivity index greater than 7 and a low toxicity. Our results provide a basis for further scaffold optimization and structure-based drug design aimed at the identification of potent anti-trypanosomatidic compounds targeting multiple PTR1 variants.
Crystal structure of 1'-ethyl-spiro[chroman-4,4'-imidazolidine]-2',5'-dione: a hydantoine derivative.[Pubmed:26594433]
Acta Crystallogr E Crystallogr Commun. 2015 Sep 12;71(Pt 10):o705-6.
The title compound, C13H13N2O3, a hydantoin derivative, crystallized with two mol-ecules (A and B) in an asymmetric unit. In mol-ecule A, the imidazolidine ring is twisted about the C-N bond involving the spiro C atom, while in mol-ecule B this ring is flat (r.m.s. deviation = 0.010 A). The pyran rings in both mol-ecules have distorted half-chair conformations. The mean plane of the imidazolidine ring is inclined to the aromatic ring of the chroman unit by 79.71 (11) degrees in mol-ecule A and 82.83 (12) degrees in mol-ecule B. In the crystal, pairs of N-Hcdots, three dots, centeredO hydrogen bonds link the individual mol-ecules to form A-A and B-B inversion dimers. The dimers are linked via N-Hcdots, three dots, centeredO and C-Hcdots, three dots, centeredO hydrogen bonds, forming sheets lying parallel to the bc plane, viz. (011). Within the sheets, the A and B mol-ecules are linked by C-Hcdots, three dots, centeredpi inter-actions.
Crystal structure determination as part of an undergraduate laboratory experiment: 1',3',3'-tri-methyl-spiro-[chromene-2,2'-indoline] and 1',3',3'-trimethyl-4-[(E)-(1,3,3-tri-methyl-indolin-2-yl-idene)meth-yl]spiro-[chr oman-2,2'-indoline].[Pubmed:27840731]
Acta Crystallogr E Crystallogr Commun. 2016 Oct 28;72(Pt 11):1659-1662.
The crystal structures of the title compounds, C19H19NO and C31H34N2O, were determined as part of an experiment in an undergraduate teaching laboratory that demonstrates the relationship between mol-ecular structure and function. 1',3',3'-Tri-methyl-spiro-[chromene-2,2'-indoline] is both a photoswitch and thermochromic mol-ecule. Students synthesized it and a bis-indoline adduct and compared the crystallographically determined structures to computed gas-phase models.
Discovery of (R)-1-(7-chloro-2,2-bis(fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)ur ea (A-1165442): a temperature-neutral transient receptor potential vanilloid-1 (TRPV1) antagonist with analgesic efficacy.[Pubmed:25100568]
J Med Chem. 2014 Sep 11;57(17):7412-24.
The synthesis and characterization of a series of selective, orally bioavailable 1-(chroman-4-yl)urea TRPV1 antagonists is described. Whereas first-generation antagonists that inhibit all modes of TRPV1 activation can elicit hyperthermia, the compounds disclosed herein do not elevate core body temperature in preclinical models and only partially block acid activation of TRPV1. Advancing the SAR of this series led to the eventual identification of (R)-1-(7-chloro-2,2-bis(fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)ur ea (A-1165442, 52), an analogue that possesses excellent pharmacological selectivity, has a favorable pharmacokinetic profile, and demonstrates good efficacy against osteoarthritis pain in rodents.


