NH125Selective eEF-2 kinase inhibitor CAS# 278603-08-0 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 278603-08-0 | SDF | Download SDF |
| PubChem ID | 10436839 | Appearance | Powder |
| Formula | C27H45IN2 | M.Wt | 524.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 160 mg/mL (305.02 mM; Need ultrasonic) | ||
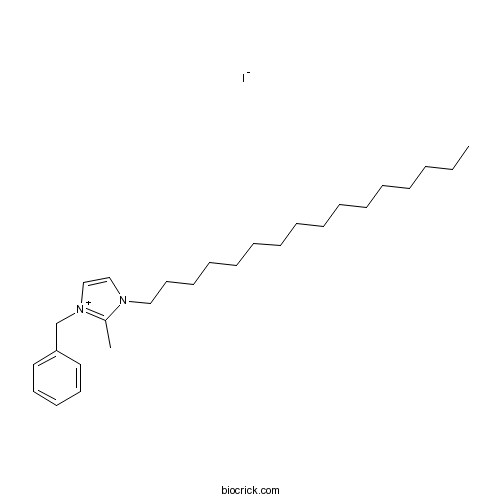
| Chemical Name | 1-benzyl-3-hexadecyl-2-methylimidazol-1-ium;iodide | ||
| SMILES | CCCCCCCCCCCCCCCCN1C=C[N+](=C1C)CC2=CC=CC=C2.[I-] | ||
| Standard InChIKey | RVWOHCBHAGBLLT-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C27H45N2.HI/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-22-28-23-24-29(26(28)2)25-27-20-17-16-18-21-27;/h16-18,20-21,23-24H,3-15,19,22,25H2,1-2H3;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Eukaryotic elongation factor 2 (eEF-2) kinase (CaMK III) inhibitor (IC50 = 60 nM) that displays 125-fold, > 1300-fold and > 1500-fold selectivity over PKC, PKA and CaMK II respectively. Decreases the viability of a variety of cancer cell lines (IC50 values are 0.7 - 4.8 μM) and blocks G1/S cell cycle progression. Shown to induce eEF-2 phosphorylation in cancer cells. Also an effective antibacterial agent; inhibits histidine protein kinase in vitro (IC50 = 6.6 μM) and in vivo. |

NH125 Dilution Calculator

NH125 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9064 mL | 9.5318 mL | 19.0636 mL | 38.1272 mL | 47.659 mL |
| 5 mM | 0.3813 mL | 1.9064 mL | 3.8127 mL | 7.6254 mL | 9.5318 mL |
| 10 mM | 0.1906 mL | 0.9532 mL | 1.9064 mL | 3.8127 mL | 4.7659 mL |
| 50 mM | 0.0381 mL | 0.1906 mL | 0.3813 mL | 0.7625 mL | 0.9532 mL |
| 100 mM | 0.0191 mL | 0.0953 mL | 0.1906 mL | 0.3813 mL | 0.4766 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NH125 is a selective inhibitor of eEF-2 with IC50 value of 60 nM [1].
Eukaryotic elongation factor-2 kinase (eEF-2 kinase or eEF-2K), also known as calcium/calmodulin-dependent eukaryotic elongation factor 2 kinase (CaMKIII) is a highly conserved protein kinase in the calmodulin-mediated signaling pathway and plays an important role in regulating protein [1, 2].
NH125 is a potent eEF-2 inhibitor and has 1000- to >100000-fold more potent against eEF-2 compared with PKC, PKA and CamK-II. When tested with a panel of 10 cancer cell lines (C6, T98-G, U-138 MG, and so forth), NH125 treatment inhibited cell viability with IC50 value ranges from 0.7 to 4.8 μM. And NH125 decreased the cellular content of p-eEF-2 without affecting total content eEF-2 and arrested cell in G0-G1 phase in C6 glioma cells [1]. In HUVECs, NH125 treatment inhibited TNF-α-induced inflammatory responses at the dose of 1μM/L [3]. When tested with a panel of human cancer cell lines (glioblastoma, breast cancer, and so on), NH125 sensitized cells at the dose of 0.25 μM which thus reinforced the efficacy of ER stress-inducing drug by inhibiting eEF-2 [2].
In spontaneously hypertensive-SHR (10 wk old) rat model, administration of NH125 (500 μg·kg−1·day−1) for 6 weeks resulted in significant reduction SBP, reduced the increased expression of VCAM-1 and E-selectin and inhibited the increased ROS production and wall thickness in SHR [3].
It is also reported that NH125 inhibited protein kinase C (PKC), protein kinase A (PKA), and calmodulin-dependent kinase II (CamK-II) with IC50 value of 7.5 μM, 80 μM and > 100 μM, respectively [1].
References:
[1]. Arora, S., et al., Identification and characterization of an inhibitor of eukaryotic elongation factor 2 kinase against human cancer cell lines. Cancer Res, 2003. 63(20): p. 6894-9.
[2]. Cheng, Y., et al., Integrated regulation of autophagy and apoptosis by EEF2K controls cellular fate and modulates the efficacy of curcumin and velcade against tumor cells. Autophagy, 2013. 9(2): p. 208-19.
[3]. Usui, T., et al., Eukaryotic elongation factor 2 kinase regulates the development of hypertension through oxidative stress-dependent vascular inflammation. Am J Physiol Heart Circ Physiol, 2013. 305(5): p. H756-68.
- Lamiide
Catalog No.:BCN4656
CAS No.:27856-54-8
- Nicergoline
Catalog No.:BCC5214
CAS No.:27848-84-6
- Macrophylline
Catalog No.:BCN1987
CAS No.:27841-97-0
- Loxapine Succinate
Catalog No.:BCC4674
CAS No.:27833-64-3
- Serratenediol diacetate
Catalog No.:BCN5173
CAS No.:27832-84-4
- Sunset yellow
Catalog No.:BCN2222
CAS No.:2783-94-0
- UDP disodium salt
Catalog No.:BCC7570
CAS No.:27821-45-0
- Loroquine
Catalog No.:BCN2008
CAS No.:27792-82-1
- Uncarinic acid E
Catalog No.:BCN6774
CAS No.:277751-61-8
- 9-Angeloylretronecine N-oxide
Catalog No.:BCN2037
CAS No.:27773-86-0
- Ervamycine
Catalog No.:BCN5172
CAS No.:27773-39-3
- 5-Hydroxy-1-methoxyxanthone
Catalog No.:BCN6575
CAS No.:27770-13-4
- Croceic acid
Catalog No.:BCN2372
CAS No.:27876-94-4
- GW4064
Catalog No.:BCC4500
CAS No.:278779-30-9
- Bis(4-bromophenyl)acetylene
Catalog No.:BCC8882
CAS No.:2789-89-1
- H-Lys(Z)-OMe.HCl
Catalog No.:BCC2988
CAS No.:27894-50-4
- H-Glu(OBzl)-OBzl.TosOH
Catalog No.:BCC2928
CAS No.:2791-84-6
- 14-Deoxy-epsilon-caesalpin
Catalog No.:BCN7254
CAS No.:279683-46-4
- 2-Amino-3-chloro-1,4-naphthoquinone
Catalog No.:BCC8525
CAS No.:2797-51-5
- 1,6-Dibromopyrene
Catalog No.:BCC8428
CAS No.:27973-29-1
- Isosteviol
Catalog No.:BCN2685
CAS No.:27975-19-5
- Gardenin B
Catalog No.:BCN3816
CAS No.:2798-20-1
- Bellidifolin
Catalog No.:BCN7424
CAS No.:2798-25-6
- H-Cys(Trt)-OH
Catalog No.:BCC2911
CAS No.:2799-07-7
NH125 reduces the level of CPEB3, an RNA binding protein, to promote synaptic GluA2 expression.[Pubmed:25842244]
Neuropharmacology. 2016 Feb;101:531-7.
Neuronal activity can alter the phosphorylation state of eukaryotic elongation factor 2 (eEF2) and thereby regulates protein synthesis. This is thought to be the underlying mechanism for a form of synaptic plasticity that involves changes in the expression of synaptic AMPA type glutamate receptors. Phosphorylation of eEF2 by Ca/calmodulin-dependent eEF2 kinase reduces the activity of eEF2, and this is prevented by a commonly used eEF2 kinase inhibitor, NH125. Here we show that 10 muM NH125 increased the expression of synaptic GluA2-containing receptors in mouse cerebellar stellate cells and this was prevented by a protein synthesis inhibitor. However NH125 at 10 muM also reduced the level of CPEB3, a protein that is known to bind to GluA2 mRNA and suppress GluA2 (also known as GluR2) synthesis. In contrast, a low concentration of NH125 lowered the peEF2 level, but did not alter CPEB3 expression and also failed to increase synaptic GluA2 receptors. A selective eEF2 kinase inhibitor, A-484954, decreased the level of peEF2, without changing the expression of CPEB3. This suggests that reducing peEF2 does not lead to a decrease in CPEB3 levels and is not sufficient to increase GluA2 synthesis. Thus NH125 at 10 muM reduced the level of CPEB3, and promoted GluA2 translation via a mechanism independent of inhibition of eEF2 kinase. Therefore NH125 does not always alter protein synthesis via selective inhibition of eEF2 kinase and the effects of NH125 on translation of mRNAs should be interpreted with caution.
Investigating the kinetic mechanism of inhibition of elongation factor 2 kinase by NH125: evidence of a common in vitro artifact.[Pubmed:22352903]
Biochemistry. 2012 Mar 13;51(10):2100-12.
Evidence that elongation factor 2 kinase (eEF-2K) has potential as a target for anticancer therapy and possibly for the treatment of depression is emerging. Here the steady-state kinetic mechanism of eEF-2K is presented using a peptide substrate and is shown to conform to an ordered sequential mechanism with ATP binding first. Substrate inhibition by the peptide was observed and revealed to be competitive with ATP, explaining the observed ordered mechanism. Several small molecules are reported to inhibit eEF-2K activity with the most notable being the histidine kinase inhibitor NH125, which has been used in a number of studies to characterize eEF-2K activity in cells. While NH125 was previously reported to inhibit eEF-2K in vitro with an IC(50) of 60 nM, its mechanism of action was not established. Using the same kinetic assay, the ability of an authentic sample of NH125 to inhibit eEF-2K was assessed over a range of substrate and inhibitor concentrations. A typical dose-response curve for the inhibition of eEF-2K by NH125 is best fit to an IC(50) of 18 +/- 0.25 muM and a Hill coefficient of 3.7 +/- 0.14, suggesting that NH125 is a weak inhibitor of eEF-2K under the experimental conditions of a standard in vitro kinase assay. To test the possibility that NH125 is a potent inhibitor of eEF2 phosphorylation, we assessed its ability to inhibit the phosphorylation of eEF2. Under standard kinase assay conditions, NH125 exhibits a similar weak ability to inhibit the phosphorylation of eEF2 by eEF-2K. Notably, the activity of NH125 is severely abrogated by the addition of 0.1% Triton to the kinase assay through a process that can be reversed upon dilution. These studies suggest that NH125 is a nonspecific colloidal aggregator in vitro, a notion further supported by the observation that NH125 inhibits other protein kinases, such as ERK2 and TRPM7 in a manner similar to that of eEF-2K. As NH125 is reported to inhibit eEF-2K in a cellular environment, its ability to inhibit eEF2 phosphorylation was assessed in MDA-MB-231 breast cancer, A549 lung cancer, and HEK-293T cell lines using a Western blot approach. No sign of a decrease in the level of eEF2 phosphorylation was observed up to 12 h following addition of NH125 to the media. Furthermore, contrary to the previously reported literatures, NH125 induced the phosphorylation of eEF-2.
NH125 kills methicillin-resistant Staphylococcus aureus persisters by lipid bilayer disruption.[Pubmed:26910612]
Future Med Chem. 2016;8(3):257-69.
BACKGROUND: NH125, a known WalK inhibitor kills MRSA persisters. However, its precise mode of action is still unknown. METHODS & RESULTS: The mode of action of NH125 was investigated by comparing its spectrum of antimicrobial activity and its effects on membrane permeability and giant unilamellar vesicles (GUVs) with walrycin B, a WalR inhibitor and benzyldimethylhexadecylammonium chloride (16-BAC), a cationic surfactant. NH125 killed persister cells of a variety of Staphylococcus aureus strains. Similar to 16-BAC, NH125 killed MRSA persisters by inducing rapid membrane permeabilization and caused the rupture of GUVs, whereas walrycin B did not kill MRSA persisters or induce membrane permeabilization and did not affect GUVs. CONCLUSION: NH125 kills MRSA persisters by interacting with and disrupting membranes in a detergent-like manner.
Identification of N-Arylated NH125 Analogues as Rapid Eradicating Agents against MRSA Persister Cells and Potent Biofilm Killers of Gram-Positive Pathogens.[Pubmed:27925693]
Chembiochem. 2017 Feb 16;18(4):352-357.
Bacterial biofilms housing dormant persister cells are innately tolerant to antibiotics and disinfectants, yet several membrane-active agents are known to eradicate tolerant bacterial cells. NH125, a membrane-active persister killer and starting point for development, led to the identification of two N-arylated analogues (1 and 2) that displayed improved biofilm eradication potencies compared to the parent compound and rapid persister-cell-killing activities in stationary cultures of methicillin-resistant Staphylococcus aureus (MRSA). We found 1 and 2 to be superior to other membrane-active agents in biofilm eradication assays, with 1 demonstrating minimum biofilm eradication concentrations (MBEC) of 23.5, 11.7, and 2.35 mum against MRSA, methicillin-resistant Staphylococcus epidermidis (MRSE), and vancomycin-resistant Enterococcus faecium (VRE) biofilms, respectively. We tested our panel of membrane-active agents against MRSA stationary cultures and found 1 to rapidly eradicate MRSA stationary cells by 4 log units (99.99 %) in 30 min. The potent biofilm eradication and rapid persister-cell-killing activities exhibited by N-arylated NH125 analogues could have significant impact in addressing biofilm-associated problems.
1-Benzyl-3-cetyl-2-methylimidazolium iodide (NH125) induces phosphorylation of eukaryotic elongation factor-2 (eEF2): a cautionary note on the anticancer mechanism of an eEF2 kinase inhibitor.[Pubmed:22020937]
J Biol Chem. 2011 Dec 23;286(51):43951-8.
Eukaryotic elongation factor-2 kinase (eEF2K) relays growth and stress signals to protein synthesis through phosphorylation and inactivation of eukaryotic elongation factor 2 (eEF2). 1-Benzyl-3-cetyl-2-methylimidazolium iodide (NH125) is a widely accepted inhibitor of mammalian eEF2K and an efficacious anti-proliferation agent against different cancer cells. It implied that eEF2K could be an efficacious anticancer target. However, eEF2K siRNA was ineffective against cancer cells including those sensitive to NH125. To test if pharmacological intervention differs from siRNA interference, we identified a highly selective small molecule eEF2K inhibitor A-484954. Like siRNA, A-484954 had little effect on cancer cell growth. We carefully examined the effect of NH125 and A-484954 on phosphorylation of eEF2, the known cellular substrate of eEF2K. Surprisingly, NH125 increased eEF2 phosphorylation, whereas A-484954 inhibited the phosphorylation as expected for an eEF2K inhibitor. Both A-484954 and eEF2K siRNA inhibited eEF2K and reduced eEF2 phosphorylation with little effect on cancer cell growth. These data demonstrated clearly that the anticancer activity of NH125 was more correlated with induction of eEF2 phosphorylation than inhibition of eEF2K. Actually, induction of eEF2 phosphorylation was reported to correlate with inhibition of cancer cell growth. We compared several known inducers of eEF2 phosphorylation including AMPK activators and an mTOR inhibitor. Interestingly, stronger induction of eEF2 phosphorylation correlated with more effective growth inhibition. We also explored signal transduction pathways leading to NH125-induced eEF2 phosphorylation. Preliminary data suggested that NH125-induced eEF2 phosphorylation was likely mediated through multiple pathways. These observations identified an opportunity for a new multipathway approach to anticancer therapies.
P-glycoprotein mediates resistance to histidine kinase inhibitors.[Pubmed:15322237]
Mol Pharmacol. 2004 Sep;66(3):460-7.
Histidine kinase inhibitors are being developed as a new class of antimicrobial drugs. We recently demonstrated the activity of a class of histidine kinase inhibitors against a mammalian enzyme, elongation factor-2 kinase (eEF-2K), and the effect of these compounds on cancer cell viability (Arora et al., 2003). To further characterize these compounds, we studied their interaction with ATP-binding cassette transporters, which are known to mediate resistance to a variety of chemotherapeutic agents. The 24 compounds studied belong to three structural series of derivatives of 2-methylimidazolium iodide. We focused this work on a representative compound (NH125) because we found it to be most potent against both histidine kinase and eEF-2K among the series. Cell lines that expressed P-glycoprotein (P-gp) were 2- to 5-fold resistant to NH125. NH125 increased accumulation of P-gp substrates such as paclitaxel and doxorubicin but had no effect on the accumulation of non-P-gp substrates. P-gp modulators verapamil and trans-flupenthixol and MDR1-targeted siRNA increased sensitivity of multidrug-resistant cell lines to NH125. The presence of a benzyl group on the N-3 position of the 2-methylimidazolium iodide was important for the interaction with P-gp. C6-NH, an NH125-resistant cell line, markedly overexpressed P-gp compared with the parental cell line. In animal models, we found that NH125 increased by 129% the survival of sensitive P388 cells bearing mice but had no effect on mice harboring the resistant cell line. These observations indicate that certain histidine kinase inhibitors are substrates for P-gp and hence an important consideration in development of these agents as potential antimicrobial and anticancer agents.
Identification and characterization of an inhibitor of eukaryotic elongation factor 2 kinase against human cancer cell lines.[Pubmed:14583488]
Cancer Res. 2003 Oct 15;63(20):6894-9.
Recent evidence suggests that the machinery of protein synthesis may provide novel targets for anticancer drugs. For example, aberrations in protein synthesis are commonly encountered in established cancers, and disruption by mutation or overexpression of translation factors can cause cellular transformation. We previously demonstrated that the activity of eukaryotic elongation factor 2 (eEF-2) kinase was markedly increased in several forms of malignancy and that nonspecific inhibitors of this enzyme promoted cell death. On the basis of the predicted amino acid sequence of eEF-2 kinase deduced from the cloned cDNA, we hypothesized that inhibitors of prokaryotic histidine kinases might also inhibit the activity of eEF-2 kinase. We describe herein the screening of a series of imidazolium histidine kinase inhibitors and the identification of an active lead compound, NH125. NH125 inhibited eEF-2 kinase activity (IC(50) = 60 nM) in vitro, blocked the phosphorylation of eEF-2 in intact cells, and showed relative selectivity over other protein kinases: protein kinase C (IC(50) = 7.5 microM), protein kinase A (IC(50) = 80 microM), and calmodulin-dependent kinase II (IC(50) > 100 microM). NH125 decreased the viability of 10 cancer cell lines with IC(50)s ranging from 0.7 to 4.7 microM. Forced overexpression of eEF-2 kinase in a glioma cell line produced 10-fold resistance to NH125. In conclusion, these results suggest that identification of potent inhibitors of eEF-2 kinase may lead to the development of new types of anticancer drugs.
Antibacterial agents that inhibit histidine protein kinase YycG of Bacillus subtilis.[Pubmed:11758928]
Biosci Biotechnol Biochem. 2001 Oct;65(10):2306-10.
We demonstrated in vitro that YycG-YycF of Bacillus subtilis constitutes a two-component system and shows a specificity of the sensor protein for the cognate phosphorylation partner. Based on inhibition of such an autophosphorylation of YycG, we searched imidazole and zerumbone derivatives to identify the antibacterial agents such as NH125, NH126, NH127, and NH0891.


