CX-6258Pan-Pim kinases Inhibitor CAS# 1202916-90-2 |
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- LKB1 (AAK1 dual inhibitor)
Catalog No.:BCC1705
CAS No.:1093222-27-5
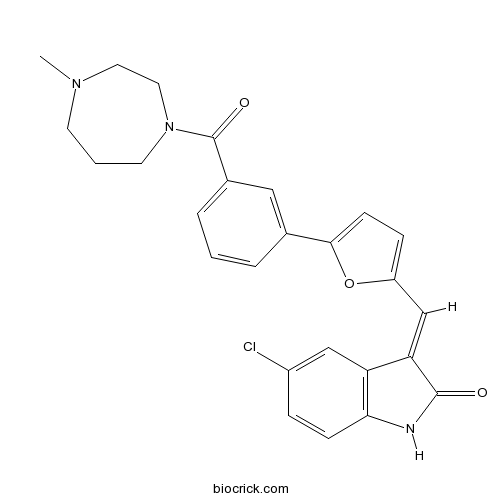
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- SMI-4a
Catalog No.:BCC2233
CAS No.:438190-29-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1202916-90-2 | SDF | Download SDF |
| PubChem ID | 44545852 | Appearance | Powder |
| Formula | C26H24ClN3O3 | M.Wt | 461.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (108.24 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (3E)-5-chloro-3-[[5-[3-(4-methyl-1,4-diazepane-1-carbonyl)phenyl]furan-2-yl]methylidene]-1H-indol-2-one | ||
| SMILES | CN1CCCN(CC1)C(=O)C2=CC=CC(=C2)C3=CC=C(O3)C=C4C5=C(C=CC(=C5)Cl)NC4=O | ||
| Standard InChIKey | KGBPLKOPSFDBOX-CJLVFECKSA-N | ||
| Standard InChI | InChI=1S/C26H24ClN3O3/c1-29-10-3-11-30(13-12-29)26(32)18-5-2-4-17(14-18)24-9-7-20(33-24)16-22-21-15-19(27)6-8-23(21)28-25(22)31/h2,4-9,14-16H,3,10-13H2,1H3,(H,28,31)/b22-16+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CX-6258 HCl is a potent, orally efficacious inhibitor of pan-Pim kinase with IC50 of 5 nM, 25 nM and 16 nM for Pim1, Pim2, and Pim3, respectively. | |||||
| Targets | Pim1 | Pim3 | Pim2 | |||
| IC50 | 5 nM | 16 nM | 25 nM | |||

CX-6258 Dilution Calculator

CX-6258 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1648 mL | 10.8239 mL | 21.6478 mL | 43.2957 mL | 54.1196 mL |
| 5 mM | 0.433 mL | 2.1648 mL | 4.3296 mL | 8.6591 mL | 10.8239 mL |
| 10 mM | 0.2165 mL | 1.0824 mL | 2.1648 mL | 4.3296 mL | 5.412 mL |
| 50 mM | 0.0433 mL | 0.2165 mL | 0.433 mL | 0.8659 mL | 1.0824 mL |
| 100 mM | 0.0216 mL | 0.1082 mL | 0.2165 mL | 0.433 mL | 0.5412 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pim kinases (Provirus Integration site for Moloney murine leukemia virus) are a family of serine/threonine kinases that regulate cell survival. This family of kinases is composed of three different isoforms (Pim-1, Pim-2, and Pim-3). The simultaneous inhibition of Pim-1, Pim-2, and Pim-3 kinases is emerging as a promising strategy for anticancer drug development. CX-6258 is a potent, selective, and orally efficacious pan-Pim kinases inhibitor.
In vitro: CX-6258 exhibited in vitro synergy with chemotherapeutics. The antiproliferative activity of CX-6258 was examined against a panel of cell lines derived from human solid tumors and hematological malignancies. CX-6258 demonstrated robust antiproliferative potencies against all cell lines tested [1].
In vivo: CX-6258 was evaluated in two human tumor xenograft growth efficacy models, acute myeloid leukemia MV-4-11, and prostate adenocarcinoma PC3. The drug exhibited dose dependent efficacy, with a 50 mg/kg dose producing 45% tumor growth inhibition (TGI) and a 100 mg/kg dose producing 75% TGI. In addition, CX-6258 displayed significant efficacy in vivo in two xenograft models representing the diseases where Pim kinases had been shown to play an important role [1].
Clinical trial: No clinical data are available
Reference:
[1] Haddach M, Michaux J, Schwaebe MK, et al. Discovery of CX-6258. A Potent, Selective, and Orally Efficacious pan-Pim Kinases Inhibitor. ACS Med Chem Lett. 2011;3(2):135-9.
- Dorzolamide
Catalog No.:BCC4287
CAS No.:120279-96-1
- 4-Hydroxysapriparaquinone
Catalog No.:BCN4806
CAS No.:120278-25-3
- Salvinolone
Catalog No.:BCN3215
CAS No.:120278-22-0
- CNX-774
Catalog No.:BCC4394
CAS No.:1202759-32-7
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- 1beta-Hydroxyeuscaphic acid
Catalog No.:BCN3517
CAS No.:120211-98-5
- Tenidap
Catalog No.:BCC7419
CAS No.:120210-48-2
- NMS-P715
Catalog No.:BCC6373
CAS No.:1202055-34-2
- Clopidogrel Related Compound C
Catalog No.:BCN2689
CAS No.:120202-71-3
- Clopidogrel
Catalog No.:BCC2497
CAS No.:120202-66-6
- 3,4-Dihydroxycinnamamide
Catalog No.:BCN6090
CAS No.:1202-41-1
- Citroside A
Catalog No.:BCN7294
CAS No.:120330-44-1
- DASA-58
Catalog No.:BCC6522
CAS No.:1203494-49-8
- Cyclotraxin B
Catalog No.:BCC6357
CAS No.:1203586-72-4
- AS 1949490
Catalog No.:BCC7762
CAS No.:1203680-76-5
- Jionoside B1
Catalog No.:BCN2858
CAS No.:120406-37-3
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- TMC647055
Catalog No.:BCC6376
CAS No.:1204416-97-6
- Verlukast
Catalog No.:BCC2035
CAS No.:120443-16-5
- Jionoside A1
Catalog No.:BCN2922
CAS No.:120444-60-2
- (±)-Vesamicol hydrochloride
Catalog No.:BCC6737
CAS No.:120447-62-3
Discovery of CX-6258. A Potent, Selective, and Orally Efficacious pan-Pim Kinases Inhibitor.[Pubmed:24900437]
ACS Med Chem Lett. 2011 Dec 27;3(2):135-9.
Structure-activity relationship analysis in a series of 3-(5-((2-oxoindolin-3-ylidene)methyl)furan-2-yl)amides identified compound 13, a pan-Pim kinases inhibitor with excellent biochemical potency and kinase selectivity. Compound 13 exhibited in vitro synergy with chemotherapeutics and robust in vivo efficacy in two Pim kinases driven tumor models.
Synthesis of [11C]CX-6258 as a new PET tracer for imaging of Pim kinases in cancer.[Pubmed:26227775]
Bioorg Med Chem Lett. 2015 Sep 15;25(18):3831-5.
The reference standard CX-6258 {(E)-5-chloro-3-((5-(3-(4-methyl-1,4-diazepane-1-carbonyl)phenyl)furan-2-yl)methy lene)indolin-2-one, 4a} and its desmethylated precursor N-desmethyl-CX-6258 {(E)-3-((5-(3-(1,4-diazepane-1-carbonyl)phenyl)furan-2-yl)methylene)-5-chloroindo lin-2-one, 5} for radiolabeling were synthesized from 5-bromo-2-furaldehyde and 3-carboxybenzeneboronic acid in 3 and 4 steps with 29-49% and 24-32% overall chemical yield, respectively. The target tracer [(11)C]CX-6258 {(E)-5-chloro-3-((5-(3-(4-[(11)C]methyl-1,4-diazepane-1-carbonyl)phenyl)furan-2-y l)methylene)indolin-2-one, [(11)C]4a} was prepared from N-desmethyl-CX-6258 (5) with [(11)C]CH3OTf under basic condition (2N NaOH) through N-[(11)C]methylation and isolated by HPLC combined with solid-phase extraction (SPE) in 40-50% radiochemical yield based on [(11)C]CO2 and decay corrected to end of bombardment (EOB) with 370-1110GBq/mumol specific activity at EOB.


