VerlukastCAS# 120443-16-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120443-16-5 | SDF | Download SDF |
| PubChem ID | 6509849 | Appearance | Powder |
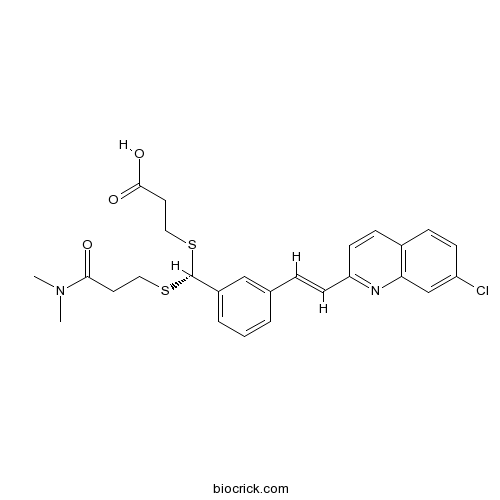
| Formula | C26H27ClN2O3S2 | M.Wt | 515.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MK-679 | ||
| Solubility | DMSO | ||
| Chemical Name | 3-[(R)-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-[3-(dimethylamino)-3-oxopropyl]sulfanylmethyl]sulfanylpropanoic acid | ||
| SMILES | CN(C)C(=O)CCSC(C1=CC=CC(=C1)C=CC2=NC3=C(C=CC(=C3)Cl)C=C2)SCCC(=O)O | ||
| Standard InChIKey | AXUZQJFHDNNPFG-LHAVAQOQSA-N | ||
| Standard InChI | InChI=1S/C26H27ClN2O3S2/c1-29(2)24(30)12-14-33-26(34-15-13-25(31)32)20-5-3-4-18(16-20)6-10-22-11-8-19-7-9-21(27)17-23(19)28-22/h3-11,16-17,26H,12-15H2,1-2H3,(H,31,32)/b10-6+/t26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Verlukast Dilution Calculator

Verlukast Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9414 mL | 9.707 mL | 19.4141 mL | 38.8282 mL | 48.5352 mL |
| 5 mM | 0.3883 mL | 1.9414 mL | 3.8828 mL | 7.7656 mL | 9.707 mL |
| 10 mM | 0.1941 mL | 0.9707 mL | 1.9414 mL | 3.8828 mL | 4.8535 mL |
| 50 mM | 0.0388 mL | 0.1941 mL | 0.3883 mL | 0.7766 mL | 0.9707 mL |
| 100 mM | 0.0194 mL | 0.0971 mL | 0.1941 mL | 0.3883 mL | 0.4854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Verlukast(MK-679; L 668019) is a receptor antagonist for the treatment of respiratory diseases.
- TMC647055
Catalog No.:BCC6376
CAS No.:1204416-97-6
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- Jionoside B1
Catalog No.:BCN2858
CAS No.:120406-37-3
- AS 1949490
Catalog No.:BCC7762
CAS No.:1203680-76-5
- Cyclotraxin B
Catalog No.:BCC6357
CAS No.:1203586-72-4
- DASA-58
Catalog No.:BCC6522
CAS No.:1203494-49-8
- Citroside A
Catalog No.:BCN7294
CAS No.:120330-44-1
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- Dorzolamide
Catalog No.:BCC4287
CAS No.:120279-96-1
- 4-Hydroxysapriparaquinone
Catalog No.:BCN4806
CAS No.:120278-25-3
- Jionoside A1
Catalog No.:BCN2922
CAS No.:120444-60-2
- (±)-Vesamicol hydrochloride
Catalog No.:BCC6737
CAS No.:120447-62-3
- ER 27319 maleate
Catalog No.:BCC5914
CAS No.:1204480-26-1
- Lophanthoidin B
Catalog No.:BCN6091
CAS No.:120462-42-2
- Lophanthoidin E
Catalog No.:BCN6092
CAS No.:120462-45-5
- Lophanthoidin F
Catalog No.:BCN6093
CAS No.:120462-46-6
- Ganoderenic acid F
Catalog No.:BCN2446
CAS No.:120462-47-7
- Ganoderenic acid H
Catalog No.:BCN2447
CAS No.:120462-48-8
- Pinanediol talabostat boronate
Catalog No.:BCC1640
CAS No.:1204669-37-3
- Epacadostat
Catalog No.:BCC6531
CAS No.:1204669-58-8
- SRT3109
Catalog No.:BCC1965
CAS No.:1204707-71-0
- SRT3190
Catalog No.:BCC1966
CAS No.:1204707-73-2
CYP1A1 specificity of Verlukast epoxidation in mice, rats, rhesus monkeys, and humans.[Pubmed:7905381]
Drug Metab Dispos. 1993 Nov-Dec;21(6):1029-36.
It has previously been shown that Verlukast is converted to Verlukast dihydrodiol in microsomes from beta-naphthoflavone (BNF)-treated, but not uninduced Swiss Webster mice and Sprague-Dawley rats. We have examined the involvement of CYP1A1 in this reaction in more detail. It is concluded that this reaction is catalyzed exclusively by CYP1A1 in rats, mice, and humans based on the following criteria: 1) the epoxidation of Verlukast is negligible in uninduced rats, which express CYP1A2 but not CYP1A1; 2) Verlukast epoxidation is highly inducible by BNF treatment (60- to 200-fold); 3) Verlukast epoxidation in BNF-treated rat microsomes was inhibited by alpha-naphthoflavone (ANF) treatment, indicating that this activity was mediated by the CYP1A subfamily; 4) > 95% of Verlukast epoxidation in BNF-treated rat microsomes was inhibited by antibodies raised against CYP1A1; and 5) Verlukast was epoxidized by human CYP1A1 but not CYP1A2. Thus, Verlukast epoxidation appears to be specific for rat, mouse, and human CYP1A1. Additional studies showed that Verlukast was metabolized to Verlukast dihydrodiol in microsomes from uninduced rhesus monkeys. This reaction was inhibited by nanomolar concentrations of ANF in rhesus monkey microsomes implicating the involvement of the CYP1A subfamily. In addition, the 8-hydroxylation of R-warfarin, a pathway that is selective for rodent and human CYP1A1 activity, was also catalyzed at significant rates by rhesus monkey microsomes. These findings indicate that, unlike rats, mice, and humans, which have very low constitutive levels of hepatic CYP1A1 activity, the uninduced rhesus monkey is able to catalyze reactions specific to CYP1A1 in rodents and humans.(ABSTRACT TRUNCATED AT 250 WORDS)
Robotic sample preparation and high-performance liquid chromatographic analysis of verlukast in human plasma.[Pubmed:7894671]
J Chromatogr B Biomed Appl. 1994 Nov 18;661(2):307-12.
A fully automated HPLC assay has been developed and validated for the quantitation of Verlukast, a leukotriene D4 antagonist, in human plasma. An upgraded Zymate I robotic system was utilized to perform protein precipitation and on-line injection followed by reversed-phase HPLC with fluorescence detection. Inter-day accuracy and precision were 100.8 and 4.6%, respectively, for the low quality control standards (0.125 microgram/ml). The automated robotic method was shown to be more efficient and accurate than the manual method.
Oral pharmacokinetics and food interaction of the leukotriene D4 receptor antagonist verlukast.[Pubmed:12959296]
Br J Clin Pharmacol. 1993 Nov;36(5):464-6.
The influence of dose and food on the pharmacokinetic profile of orally administered Verlukast, a leukotriene D4 receptor antagonist, was investigated in 12 healthy male volunteers. This was an open, four-period, single dose, randomised, crossover design including the following doses: one 75 mg tablet, one 250 mg tablet, 500 mg (2 x 250 mg) and 500 mg immediately following a standard meal. There were dose-related increases in the AUC, although after 500 mg Verlukast this was disproportionately greater than with 75 mg (P = 0.04). Similarly, there were dose-related increases in C(max). No differences were observed in the t(max) between treatments. With respect to food, there was a 22% decrease (P = 0.02) in C(max) after 500 mg, and the AUC was 13% less (P = 0.052). The differences in the plasma concentration profiles betweeen fasted and fed states are not considered to be of clinical importance.
Verlukast (MK-0679) conjugation with glutathione by rat liver and kidney cytosols and excretion in the bile.[Pubmed:8654196]
Drug Metab Dispos. 1995 Oct;23(10):1085-93.
Verlukast (MK-0679) is a potent leukotriene D4 antagonist that was under development for the treatment of bronchial asthma. A previously uncharacterized metabolite of Verlukast was formed in incubations using rat liver cytosol fortified with glutathione (GSH). The metabolite was detected by HPLC and characterized by UV spectroscopy (photodiode array detection after HPLC) and capillary HPLC continuous flow-liquid secondary-ion mass spectrometry. After a large-scale incubation and isolation, it was further characterized by 500 MHz proton NMR. The metabolite is a 1,4-Michael addition product in which GSH has added to position 12 of the styryl quinoline double bond of Verlukast. There is no apparent stereoselectivity because a mixture of the two possible isomers, in equal amounts, was observed by NMR. Although there was spontaneous chemical addition of GSH to Verlukast (0.18 nmol/min), the reaction was shown to be enzyme-catalyzed in studies using three different preparations of rat liver cytosol at pH 7.4. Using Lineweaver-Burk analysis of experiments in which the effect of Verlukast concentration on the rate of conjugation was studied, the apparent KM and Vmax were determined to be 107 +/- 22 microM (SD, N=3) and 0.66 +/- 0.21 nmol/min/mg protein, respectively. In similar studies with GSH as the variable substrate, the apparent KM and Vmax were 2.32 +/- 0.68 mM and 0.69 +/- 0.14 nmol/min/mg protein, respectively. Incubations with kidney cytosol produced the GSH, cysteinylglycine, and cysteine conjugates of Verlukast. In bile collected from rats dosed intravenously with 50 mg/kg of Verlukast, approximately 80% of the dose was recovered up to 4 hr postdose. The GSH conjugate accounted for 16.5% of the dose. The cysteinylglycine, cysteine, and N-acetylcysteine conjugates were observed and together accounted for 7.5%. Verlukast accounted for 14.5%, and the remainder of the metabolites (40.5%) were oxidation or acyl glucuronide metabolites.


