Cyclotraxin BTrkB receptor antagonist CAS# 1203586-72-4 |
- Ascomycin(FK 520)
Catalog No.:BCC1370
CAS No.:104987-12-4
- Dexamethasone acetate
Catalog No.:BCC4775
CAS No.:1177-87-3
- Azathioprine
Catalog No.:BCC4762
CAS No.:446-86-6
- Cyclosporin A
Catalog No.:BCC4773
CAS No.:59865-13-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1203586-72-4 | SDF | Download SDF |
| PubChem ID | 90489002 | Appearance | Powder |
| Formula | C48H73N13O17S3 | M.Wt | 1200.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 2 mg/ml in water | ||
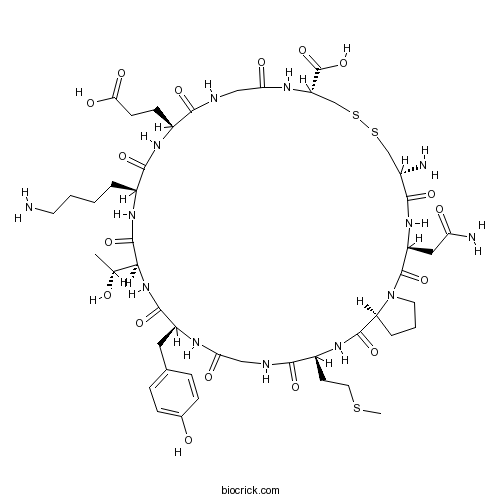
| Sequence | CNPMGYTKEGC (Modifications: Disulfide bridge between 1 - 11) | ||
| Chemical Name | (3S,6R,11R,17S,20S,23S,26S,32S,35S)-6-amino-20-(4-aminobutyl)-3-(2-amino-2-oxoethyl)-17-(2-carboxyethyl)-23-[(1R)-1-hydroxyethyl]-26-[(4-hydroxyphenyl)methyl]-32-(2-methylsulfanylethyl)-2,5,13,16,19,22,25,28,31,34-decaoxo-8,9-dithia-1,4,12,15,18,21,24,27,30,33-decazabicyclo[33.3.0]octatriacontane-11-carboxylic acid | ||
| SMILES | CC(C1C(=O)NC(C(=O)NC(C(=O)NCC(=O)NC(CSSCC(C(=O)NC(C(=O)N2CCCC2C(=O)NC(C(=O)NCC(=O)NC(C(=O)N1)CC3=CC=C(C=C3)O)CCSC)CC(=O)N)N)C(=O)O)CCC(=O)O)CCCCN)O | ||
| Standard InChIKey | JLBMMJHZUYBFGX-ZHTCEXBHSA-N | ||
| Standard InChI | InChI=1S/C48H73N13O17S3/c1-24(62)39-46(75)58-28(6-3-4-15-49)43(72)56-29(12-13-38(67)68)41(70)52-21-37(66)55-33(48(77)78)23-81-80-22-27(50)40(69)59-32(19-35(51)64)47(76)61-16-5-7-34(61)45(74)57-30(14-17-79-2)42(71)53-20-36(65)54-31(44(73)60-39)18-25-8-10-26(63)11-9-25/h8-11,24,27-34,39,62-63H,3-7,12-23,49-50H2,1-2H3,(H2,51,64)(H,52,70)(H,53,71)(H,54,65)(H,55,66)(H,56,72)(H,57,74)(H,58,75)(H,59,69)(H,60,73)(H,67,68)(H,77,78)/t24-,27+,28+,29+,30+,31+,32+,33+,34+,39+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antagonist of TrkB receptors; inhibits BDNF-induced TrkB activity (IC50 = 0.30 nM). Allosterically alters TrkB receptor conformation but does not alter BDNF binding. Prevents BDNF-induced cold allodynia in mice. Also shown to exhibit putative anxiolytic properties in mice. |

Cyclotraxin B Dilution Calculator

Cyclotraxin B Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- DASA-58
Catalog No.:BCC6522
CAS No.:1203494-49-8
- Citroside A
Catalog No.:BCN7294
CAS No.:120330-44-1
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- Dorzolamide
Catalog No.:BCC4287
CAS No.:120279-96-1
- 4-Hydroxysapriparaquinone
Catalog No.:BCN4806
CAS No.:120278-25-3
- Salvinolone
Catalog No.:BCN3215
CAS No.:120278-22-0
- CNX-774
Catalog No.:BCC4394
CAS No.:1202759-32-7
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- 1beta-Hydroxyeuscaphic acid
Catalog No.:BCN3517
CAS No.:120211-98-5
- Tenidap
Catalog No.:BCC7419
CAS No.:120210-48-2
- NMS-P715
Catalog No.:BCC6373
CAS No.:1202055-34-2
- AS 1949490
Catalog No.:BCC7762
CAS No.:1203680-76-5
- Jionoside B1
Catalog No.:BCN2858
CAS No.:120406-37-3
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- TMC647055
Catalog No.:BCC6376
CAS No.:1204416-97-6
- Verlukast
Catalog No.:BCC2035
CAS No.:120443-16-5
- Jionoside A1
Catalog No.:BCN2922
CAS No.:120444-60-2
- (±)-Vesamicol hydrochloride
Catalog No.:BCC6737
CAS No.:120447-62-3
- ER 27319 maleate
Catalog No.:BCC5914
CAS No.:1204480-26-1
- Lophanthoidin B
Catalog No.:BCN6091
CAS No.:120462-42-2
- Lophanthoidin E
Catalog No.:BCN6092
CAS No.:120462-45-5
Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice.[Pubmed:20333308]
PLoS One. 2010 Mar 19;5(3):e9777.
In the last decades, few mechanistically novel therapeutic agents have been developed to treat mental and neurodegenerative disorders. Numerous studies suggest that targeting BDNF and its TrkB receptor could be a promising therapeutic strategy for the treatment of brain disorders. However, the development of potent small ligands for the TrkB receptor has proven to be difficult. By using a peptidomimetic approach, we developed a highly potent and selective TrkB inhibitor, cyclotraxin-B, capable of altering TrkB-dependent molecular and physiological processes such as synaptic plasticity, neuronal differentiation and BDNF-induced neurotoxicity. Cyclotraxin-B allosterically alters the conformation of TrkB, which leads to the inhibition of both BDNF-dependent and -independent (basal) activities. Finally, systemic administration of cyclotraxin-B to mice results in TrkB inhibition in the brain with specific anxiolytic-like behavioral effects and no antidepressant-like activity. This study demonstrates that cyclotraxin-B might not only be a powerful tool to investigate the role of BDNF and TrkB in physiology and pathology, but also represents a lead compound for the development of new therapeutic strategies to treat brain disorders.
Cyclotraxin-B, a new TrkB antagonist, and glial blockade by propentofylline, equally prevent and reverse cold allodynia induced by BDNF or partial infraorbital nerve constriction in mice.[Pubmed:22560237]
J Pain. 2012 Jun;13(6):579-89.
UNLABELLED: Several lines of evidence indicate that brain-derived neurotrophic factor (BDNF) plays a key role as a central pronociceptive modulator of pain, acting through postsynaptic TrkB receptors that trigger intracellular signaling cascades leading to central sensitization. The overall aim of this study was to investigate to what extent BDNF could participate in the generation and maintenance of trigeminal neuropathic pain. The results showed that acute intracisternal administration of nanogram doses of BDNF in naive mice elicited long-lasting, dose-related, cold allodynic responses to topical application of acetone onto vibrissal pad skin. The systemic administration of cyclotraxin-B (CTX-B), a new TrkB receptor antagonist, or propentofylline, an inhibitor of glial activation, was able to either prevent or reverse the effects of intracisternal BDNF on cold nociception. In addition, the blockade of TrkB receptor by CTX-B inhibited the mechanisms that either initiate or maintain cold allodynia in the ipsilateral vibrissal pad skin after unilateral constriction of the infraorbital nerve. These observations raise the possibility that BDNF is capable on its own of conveying many features of the signaling mechanisms that underlie central sensitization caused by nerve constriction. PERSPECTIVE: Although further studies are necessary to examine in detail the mechanisms underlying the strong anti-allodynic action of CTX-B, this compound may represent an interesting lead for the development of novel therapeutic strategies aimed at preventing and/or suppressing central sensitization associated with neuropathic pain.


