(±)-Vesamicol hydrochlorideInhibits ACh transport CAS# 120447-62-3 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- MRK 560
Catalog No.:BCC2345
CAS No.:677772-84-8
- PF-03084014
Catalog No.:BCC1848
CAS No.:865773-15-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120447-62-3 | SDF | Download SDF |
| PubChem ID | 212231 | Appearance | Powder |
| Formula | C17H26ClNO | M.Wt | 295.85 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AH 5183 | ||
| Solubility | Soluble to 20 mM in ethanol and to 50 mM in DMSO with gentle warming | ||
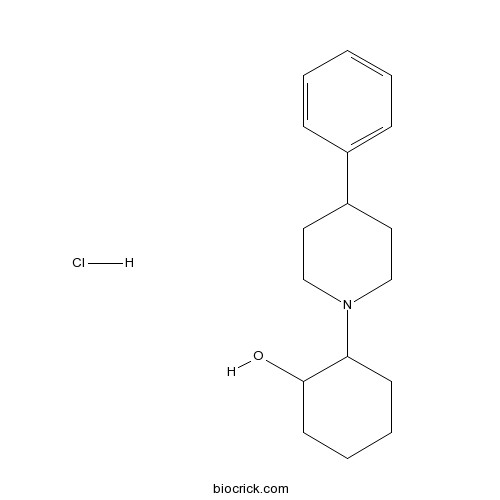
| Chemical Name | 2-(4-phenylpiperidin-1-yl)cyclohexan-1-ol;hydrochloride | ||
| SMILES | C1CCC(C(C1)N2CCC(CC2)C3=CC=CC=C3)O.Cl | ||
| Standard InChIKey | XJNUHVMJVWOYCW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H25NO.ClH/c19-17-9-5-4-8-16(17)18-12-10-15(11-13-18)14-6-2-1-3-7-14;/h1-3,6-7,15-17,19H,4-5,8-13H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of acetylcholine transport (Ki = 2 nM). Centrally active following systemic administration in vivo. |

(±)-Vesamicol hydrochloride Dilution Calculator

(±)-Vesamicol hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3801 mL | 16.9005 mL | 33.8009 mL | 67.6018 mL | 84.5023 mL |
| 5 mM | 0.676 mL | 3.3801 mL | 6.7602 mL | 13.5204 mL | 16.9005 mL |
| 10 mM | 0.338 mL | 1.69 mL | 3.3801 mL | 6.7602 mL | 8.4502 mL |
| 50 mM | 0.0676 mL | 0.338 mL | 0.676 mL | 1.352 mL | 1.69 mL |
| 100 mM | 0.0338 mL | 0.169 mL | 0.338 mL | 0.676 mL | 0.845 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Jionoside A1
Catalog No.:BCN2922
CAS No.:120444-60-2
- Verlukast
Catalog No.:BCC2035
CAS No.:120443-16-5
- TMC647055
Catalog No.:BCC6376
CAS No.:1204416-97-6
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- Jionoside B1
Catalog No.:BCN2858
CAS No.:120406-37-3
- AS 1949490
Catalog No.:BCC7762
CAS No.:1203680-76-5
- Cyclotraxin B
Catalog No.:BCC6357
CAS No.:1203586-72-4
- DASA-58
Catalog No.:BCC6522
CAS No.:1203494-49-8
- Citroside A
Catalog No.:BCN7294
CAS No.:120330-44-1
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- ER 27319 maleate
Catalog No.:BCC5914
CAS No.:1204480-26-1
- Lophanthoidin B
Catalog No.:BCN6091
CAS No.:120462-42-2
- Lophanthoidin E
Catalog No.:BCN6092
CAS No.:120462-45-5
- Lophanthoidin F
Catalog No.:BCN6093
CAS No.:120462-46-6
- Ganoderenic acid F
Catalog No.:BCN2446
CAS No.:120462-47-7
- Ganoderenic acid H
Catalog No.:BCN2447
CAS No.:120462-48-8
- Pinanediol talabostat boronate
Catalog No.:BCC1640
CAS No.:1204669-37-3
- Epacadostat
Catalog No.:BCC6531
CAS No.:1204669-58-8
- SRT3109
Catalog No.:BCC1965
CAS No.:1204707-71-0
- SRT3190
Catalog No.:BCC1966
CAS No.:1204707-73-2
- Desacetyldoronine
Catalog No.:BCN2105
CAS No.:120481-77-8
- Anastrozole
Catalog No.:BCC4370
CAS No.:120511-73-1
N-hydroxyalkyl derivatives of 3 beta-phenyltropane and 1-methylspiro[1H-indoline-3,4'-piperidine]: vesamicol analogues with affinity for monoamine transporters.[Pubmed:9397171]
J Med Chem. 1997 Nov 21;40(24):3905-14.
As part of our ongoing structure-activity studies of the vesicular acetylcholine transporter ligand 2-(4-phenylpiperidino)cyclohexanol (vesamicol, 1), 22 N-hydroxy(phenyl)alkyl derivatives of 3 beta-phenyltropane, 6, and 1-methylspiro[1H-indoline-3,4'-piperidine], 7, were synthesized and tested for binding in vitro. Although a few compounds displayed moderately high affinity for the vesicular acetylcholine transporter, no compound was more potent than the prototypical vesicular acetylcholine transporter ligand vesamicol. However, a few derivatives of 6 displayed higher affinity for the dopamine transporter than cocaine. We conclude that modification of the piperidyl fragment of 1 will not lead to more potent vesicular acetylcholine transporter ligands.
Effects of systemic administration of 2-(4-phenyl-piperidino)-cyclohexanol (vesamicol) and an organophosphate DDVP on the cholinergic system in brain regions of rats.[Pubmed:9205789]
Brain Res Bull. 1997;43(1):17-23.
Vesamicol is known to inhibit the transport of acetylcholine (ACh) into synaptic vesicles in vitro, but much less is known about its effects in the brain in vivo. To assess the effect of vesamicol in vivo, we examined cholinergic parameters, such as the subcellular distribution of ACh, activities of enzymes, uptake of choline, and muscarinic receptor binding in the striatum, hippocampus, and cerebral cortex of rats 30 and 60 min after intraperitoneal injection of vesamicol (3 mg/kg) or of vesamicol in combination with DDVP (5 mg/kg), which was administered 10 min before vasamicol. The levels of cytosolic ACh increased in all regions of the brain after injection of vesamicol, while those of vesicular ACh decreased in all regions except for the striatum. The increase in the levels of extracellular ACh and cytosolic ACh in the striatum induced by DDVP was generally enhanced after injection of vesamicol, Vesamicol did not reduce the level of vesicular ACh when DDVP had been injected previously. Vesamicol did not induce any significant changes in the activities of enzymes, choline uptake, or binding of [6H]quinuclidinyl benzilate to the muscarinic ACh receptors in the three regions. Changes in the cholinergic parameters caused by DDVP were not reversed by the combined administration of DDVP with vesamicol. The present results indicate that vesamicol can inhibit the transport of ACh into synaptic vesicles in the brain tissue in vivo, although it cannot reverse the effects of DDVP that has been injected prior to vesamicol.
Active transport of acetylcholine by the human vesicular acetylcholine transporter.[Pubmed:8910293]
J Biol Chem. 1996 Nov 1;271(44):27229-32.
The characteristics of ATP-dependent transport of acetylcholine (ACh) in homogenates of pheochromocytoma (PC-12) cells stably transfected with the human vesicular acetylcholine transporter (VAChT) cDNA are described. The human VAChT protein was abundantly expressed in this line and appeared as a diffuse band with a molecular mass of approximately 75 kDa on Western blots. Vesicular [3H]ACh accumulation increased approximately 20 times over levels attained by the endogenous rat VAChT, expressed at low levels in control PC-12 cells. The transport of [3H]ACh by human VAChT was dependent upon the addition of exogenous ATP at 37 degrees C. Uptake was abolished by low temperature (4 degrees C), the proton ionophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone (2.5 microM) and bafilomycin A1 (1 microM), a specific inhibitor of the vesicular H+-ATPase. The kinetics of [3H]ACh uptake by human VAChT were saturable, exhibiting an apparent Km of 0.97 +/- 0.1 mM and Vmax of 0.58 +/- 0.04 nmol/min/mg. Maximal steady-state levels of vesicular [3H]ACh accumulation were directly proportional to the concentration of substrate present in the medium with saturation occurring at approximately 4 mM. Uptake was stereospecifically inhibited by L-vesamicol with an IC50 of 14.7 +/- 1.5 nM. The apparent affinity (Kd) of [3H]vesamicol for human VAChT was 4.1 +/- 0.5 nM, and the Bmax was 8.9 +/- 0.6 pmol/mg. The turnover (Vmax/Bmax) of the human VAChT was approximately 65/min. This expression system should prove useful for the structure/function analysis of VAChT.


