PF-03084014γ-secretase inhibitor CAS# 865773-15-5 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- BMS 299897
Catalog No.:BCC2340
CAS No.:290315-45-6
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- MK-0752
Catalog No.:BCC2090
CAS No.:471905-41-6
- Begacestat
Catalog No.:BCC2346
CAS No.:769169-27-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 865773-15-5 | SDF | Download SDF |
| PubChem ID | 46224413 | Appearance | Powder |
| Formula | C27H41F2N5O | M.Wt | 489.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >19.8mg/mL in DMSO | ||
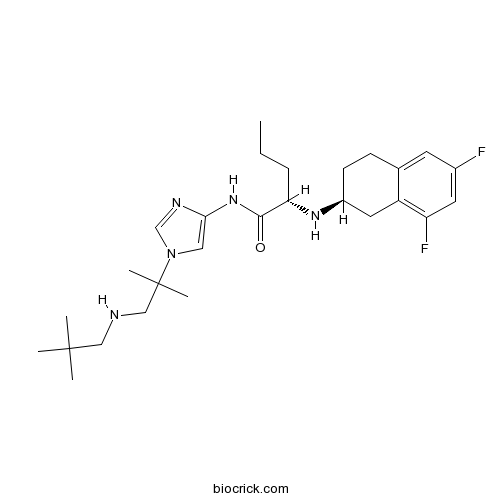
| Chemical Name | (2S)-2-[[(2S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl]amino]-N-[1-[1-(2,2-dimethylpropylamino)-2-methylpropan-2-yl]imidazol-4-yl]pentanamide | ||
| SMILES | CCCC(C(=O)NC1=CN(C=N1)C(C)(C)CNCC(C)(C)C)NC2CCC3=CC(=CC(=C3C2)F)F | ||
| Standard InChIKey | VFCRKLWBYMDAED-REWPJTCUSA-N | ||
| Standard InChI | InChI=1S/C27H41F2N5O/c1-7-8-23(32-20-10-9-18-11-19(28)12-22(29)21(18)13-20)25(35)33-24-14-34(17-31-24)27(5,6)16-30-15-26(2,3)4/h11-12,14,17,20,23,30,32H,7-10,13,15-16H2,1-6H3,(H,33,35)/t20-,23-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

PF-03084014 Dilution Calculator

PF-03084014 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0423 mL | 10.2116 mL | 20.4232 mL | 40.8463 mL | 51.0579 mL |
| 5 mM | 0.4085 mL | 2.0423 mL | 4.0846 mL | 8.1693 mL | 10.2116 mL |
| 10 mM | 0.2042 mL | 1.0212 mL | 2.0423 mL | 4.0846 mL | 5.1058 mL |
| 50 mM | 0.0408 mL | 0.2042 mL | 0.4085 mL | 0.8169 mL | 1.0212 mL |
| 100 mM | 0.0204 mL | 0.1021 mL | 0.2042 mL | 0.4085 mL | 0.5106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PF-03084014 is a reversible and selective inhibitor of γ-secretase with IC50 value of 6.2 nM [1].
The Notch signaling pathway is triggered by the interaction of Notch ligand and Notch receptor located in the membrane of the adjacent cell. Then the NICD fragment is released through the cleavage caused by γ-secretase and subsequently regulates the transcription of the downstream genes. Since Notch signaling pathway appears to be important in the development of many cancers, the small-molecule inhibitors of γ-secretase are now developed as antitumor drugs in cancer treatment. PF-03084014 is one of these GSIs (γ-secretase inhibitors) that showed IC50 value of 6.2 nM in inhibiting the production of Aβ in HeLa cells. Meanwhile PF-03084014 has no significant inhibition effect on other receptors, proteases, ion channels and kinases, demonstrating its selectivity against γ-secretase [1, 2].
In CLL (chronic lymphocytic leukemia) cells from mutated CLL patients, the treatment of 10 μM PF-03084014 induced 29.63% apoptosis. PF-03084014 at concentration of 1 μM induced cell apoptosis with 7.84%. In Notch1-unmutated cells, PF-03084014 induced apoptosis with 22.04% and 4.44% at concentrations of 1 and 10 μM, respectively. PF-03084014 showed no significant apoptosis induction in normal T cells from Notch1-mutated CLL patients. In HPB-ALL cells harboring Notch1 mutations, PF-03084014 inhibited Notch receptor cleavage with IC50 value of 13.3 nM. It also down-regulated the expressions of Hes-1 and cMyc in the cells. It is found that PF-03084014 inhibits cell growth through induce cell cycle arrest at G0-G1 phase [1, 3].
In HPB-ALL tumor xenograft model, oral administration of PF-03084014 at dose of 50 mg/kg inhibited NICD production (70%-80%) after 24 hours. 150 mg/kg PF-03084014 caused maximal tumor growth inhibition of 92%. Besides that, the combination treatment of PF-03084014 and GEM significantly inhibited tumor growth and caused tumor regression in implanted PDA (pancreatic ductal adenocarcinoma) xenografts. It is also reported that the coadministration of PF-03084014 and dexamethasone can abrogate the gastrointestinal toxicity induced by PF-03084014 [1, 4].
References:
1.Wei P, Walls M, Qiu M, et al. Evaluation of selective γ-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Molecular cancer therapeutics, 2010, 9(6): 1618-1628.
2.Arcaroli J J, Quackenbush K S, Purkey A, et al. Tumours with elevated levels of the Notch and Wnt pathways exhibit efficacy to PF-03084014, a γ-secretase inhibitor, in a preclinical colorectal explant model. British journal of cancer, 2013, 109(3): 667-675.
3.López-Guerra M, Xargay-Torrent S, Rosich L, et al. The γ-secretase inhibitor PF-03084014 combined with fludarabine antagonizes migration, invasion and angiogenesis in NOTCH1-mutated CLL cells. Leukemia, 2014.
4.Yabuuchi S, Pai S G, Campbell N R, et al. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer letters, 2013, 335(1): 41-51.
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
- 2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile
Catalog No.:BCC8506
CAS No.:865758-96-9
- TC-E 5001
Catalog No.:BCC6355
CAS No.:865565-29-3
- Ganoderic acid SZ
Catalog No.:BCN4413
CAS No.:865543-37-9
- Junipediol A
Catalog No.:BCN6912
CAS No.:86548-91-6
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Bisisorhapontigenin A
Catalog No.:BCN3501
CAS No.:865474-98-2
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- apigenin 7-O-(6〃-O-malonyl)-β-D-glucoside
Catalog No.:BCN8399
CAS No.:86546-87-4
- Benazepril
Catalog No.:BCC4286
CAS No.:86541-75-5
- Benazepril HCl
Catalog No.:BCC5019
CAS No.:86541-74-4
- N-Methylcalycinine
Catalog No.:BCN4412
CAS No.:86537-66-8
- Cytochrome c - pigeon (88-104)
Catalog No.:BCC1038
CAS No.:86579-06-8
- RX-3117
Catalog No.:BCC6381
CAS No.:865838-26-2
- Chamaejasmenin D
Catalog No.:BCN3046
CAS No.:865852-47-7
- Isochamaejasmenin B
Catalog No.:BCN3045
CAS No.:865852-48-8
- Tideglusib
Catalog No.:BCC4511
CAS No.:865854-05-3
- Oleuropeic acid 8-O-glucoside
Catalog No.:BCN4025
CAS No.:865887-46-3
- L-745,870 trihydrochloride
Catalog No.:BCC5695
CAS No.:866021-03-6
- 6-Benzoyl-5,7-dihydroxy-2,2-dimethylchromane
Catalog No.:BCN1326
CAS No.:86606-14-6
- Clausine Z
Catalog No.:BCN4414
CAS No.:866111-14-0
- PRX-08066
Catalog No.:BCC4209
CAS No.:866206-54-4
- PRX-08066 Maleic acid
Catalog No.:BCC1165
CAS No.:866206-55-5
- 3,4,5-Tricaffeoylquinic acid
Catalog No.:BCN2384
CAS No.:86632-03-3
Clinical Activity of the gamma-Secretase Inhibitor PF-03084014 in Adults With Desmoid Tumors (Aggressive Fibromatosis).[Pubmed:28350521]
J Clin Oncol. 2017 May 10;35(14):1561-1569.
Purpose Desmoid tumors (aggressive fibromatosis) arise from connective tissue cells or fibroblasts. In general, they are slow growing and do not metastasize; however, locally aggressive desmoid tumors can cause severe morbidity and loss of function. Disease recurrence after surgery and/or radiation and diagnosis of multifocal desmoid tumors highlight the need to develop effective systemic treatments for this disease. In this study, we evaluate objective response rate after therapy with the gamma-secretase inhibitor PF-03084014 in patients with recurrent, refractory, progressive desmoid tumors. Patients and Methods Seventeen patients with desmoid tumors received PF-03084014 150 mg orally twice a day in 3-week cycles. Response to treatment was evaluated at cycle 1 and every six cycles, that is, 18 weeks, by RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1. Patient-reported outcomes were measured at baseline and at every restaging visit by using the MD Anderson Symptoms Inventory. Archival tumor and blood samples were genotyped for somatic and germline mutations in APC and CTNNB1. Results Of 17 patients accrued to the study, 15 had mutations in APC or CTNNB1 genes. Sixteen patients (94%) were evaluable for response; five (29%) experienced a confirmed partial response and have been on study for more than 2 years. Another five patients with prolonged stable disease as their best response remain on study. Patient-reported outcomes confirmed clinician reporting that the investigational agent was well tolerated and, in subgroup analyses, participants who demonstrated partial response also experienced clinically meaningful and statistically significant improvements in symptom burden. Conclusion PF-03084014 was well tolerated and demonstrated promising clinical benefit in patients with refractory, progressive desmoid tumors who receive long-term treatment.
Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer.[Pubmed:27906684]
Oncotarget. 2017 Jan 10;8(2):2320-2328.
BACKGROUND: The NOTCH signaling pathway may be involved in the survival of stem cell-like tumor-initiating cells and contribute to tumor growth. In this phase Ib, open-label, multicenter study (NCT01876251), we evaluated PF-03084014, a selective gamma-secretase inhibitor in patients with advanced triple-negative breast cancer. METHODS: The dose-finding part was based on a 2x3 matrix design using the modified toxicity probability interval method. Oral PF-03084014 was administered twice daily continuously in combination with intravenous docetaxel given on day 1 of each 21-day cycle. Primary endpoint was first-cycle dose-limiting toxicity (DLT) for the dose-finding part and 6-month progression-free survival (PFS) for the expansion cohort treated at the maximum tolerated dose (MTD). Secondary endpoints included safety, objective response, and pharmacokinetics of the combination. RESULTS AND CONCLUSIONS: The MTD was estimated to be PF-03084014 100 mg twice daily / docetaxel 75 mg/m2. At this dose level, combination treatment was generally well tolerated (one DLT, grade 3 diarrhea, among eight DLT-evaluable patients). The most common all-grade, treatment-related adverse events reported in all patients (N = 29) were neutropenia (90%), fatigue (79%), nausea (72%), leukopenia (69%), diarrhea (59%), alopecia (55%), anemia (55%), and vomiting (48%). No effect was observed on the pharmacokinetics of docetaxel when administered in combination with PF-03084014. Four (16%) of 25 response-evaluable patients achieved a confirmed partial response; nine (36%) patients had stable disease, including five patients with unconfirmed partial response. In the expansion cohort, median PFS was 4.1 (95% CI 1.3-8.1) months (6-month PFS rate 17.1% [95% CI 0.8-52.6%]).
Notch Pathway Inhibition Using PF-03084014, a gamma-Secretase Inhibitor (GSI), Enhances the Antitumor Effect of Docetaxel in Prostate Cancer.[Pubmed:26202948]
Clin Cancer Res. 2015 Oct 15;21(20):4619-29.
PURPOSE: To investigate the efficacy and mechanisms of Notch signaling inhibition as an adjuvant to docetaxel in castration-resistant prostate cancer (CRPC) using a gamma-secretase inhibitor (GSI), PF-03084014. EXPERIMENTAL DESIGN: The effect of PF-03084014 on response to docetaxel was evaluated in docetaxel-sensitive and docetaxel-resistant CRPC cell lines in vitro and in murine models. Both soft tissue and bone sites were evaluated in vivo. Impacts on cell proliferation, apoptosis, cancer stem cells, and angiogenesis were evaluated. RESULTS: The combination of PF-03084014 plus docetaxel reduced both docetaxel-sensitive and docetaxel-resistant CRPC tumor growth in soft tissue and bone greater than either agent alone. Antitumor activity was associated with PF-03084014-induced inhibition of Notch pathway signaling; decreased survival signals (cyclin E; MEK/ERK, PI3K/AKT, EGFR and NF-kappaB pathway; BCL-2, BCL-XL); increased apoptotic signals (BAK, BAX; cleaved caspase-3); reduced microvessel density; reduced epithelial-mesenchymal transition; and reduced cancer stem-like cells in the tumor. CONCLUSIONS: These results reveal that PF-03084014 enhances docetaxel-mediated tumor response and provides a rationale to explore GSIs as adjunct therapy in conjunction with docetaxel for men with CRPC.


