TrelagliptinDPP-4 inhibitor,long-acting and selective CAS# 865759-25-7 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 865759-25-7 | SDF | Download SDF |
| PubChem ID | 15983988 | Appearance | Powder |
| Formula | C18H20FN5O2 | M.Wt | 357.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SYR-472 | ||
| Solubility | DMSO : ≥ 50 mg/mL (139.91 mM) *"≥" means soluble, but saturation unknown. | ||
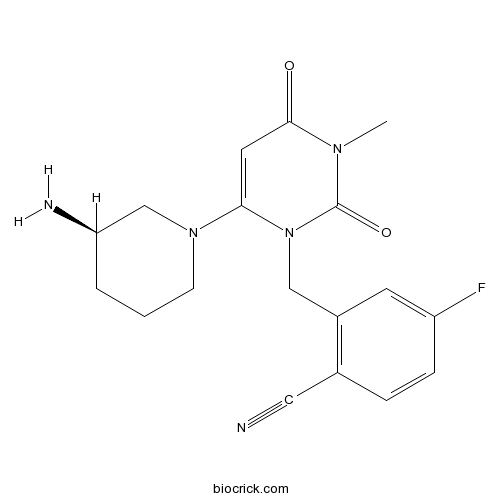
| Chemical Name | 2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]-4-fluorobenzonitrile | ||
| SMILES | CN1C(=O)C=C(N(C1=O)CC2=C(C=CC(=C2)F)C#N)N3CCCC(C3)N | ||
| Standard InChIKey | IWYJYHUNXVAVAA-OAHLLOKOSA-N | ||
| Standard InChI | InChI=1S/C18H20FN5O2/c1-22-17(25)8-16(23-6-2-3-15(21)11-23)24(18(22)26)10-13-7-14(19)5-4-12(13)9-20/h4-5,7-8,15H,2-3,6,10-11,21H2,1H3/t15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Trelagliptin is a long acting inhibitor of dipeptidyl peptidase-4 (DPP-4). | |||||
| Targets | DPP-4 | |||||

Trelagliptin Dilution Calculator

Trelagliptin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7981 mL | 13.9907 mL | 27.9814 mL | 55.9628 mL | 69.9536 mL |
| 5 mM | 0.5596 mL | 2.7981 mL | 5.5963 mL | 11.1926 mL | 13.9907 mL |
| 10 mM | 0.2798 mL | 1.3991 mL | 2.7981 mL | 5.5963 mL | 6.9954 mL |
| 50 mM | 0.056 mL | 0.2798 mL | 0.5596 mL | 1.1193 mL | 1.3991 mL |
| 100 mM | 0.028 mL | 0.1399 mL | 0.2798 mL | 0.5596 mL | 0.6995 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Trelagliptin (SYR-472) is a selective inhibitor of DPP-4 and is developed for the treatment of type 2 diabetes mellitus (T2DM) [1].
Dipeptidyl peptidase (DPP)-4 also known as adenosine deaminase complexing protein 2 or CD26 is an antigenic enzyme that expressed on the surface of most cell types and plays an important role in regulating immune system, signal transduction and apoptosis [2, 3].
Trelagliptin (SYR-472) is a potent DPP-4 inhibitor and can be administered once weekly which unlike its other class approved agents that be taken once daily [1]. In Japan, clinical trials had been conducted to detect the effectiveness of Trelagliptin by comparing with the approved medicine—alogliptin and it was shown that Trelagliptin once-weekly exhibited similar efficacy and safety to alogliptin once daily, which indicated that Trelagliptin could be a useful new antidiabetes drug that needs to be given once a week [2].
Trelagliptin (SYR-472) has been used in phase 2 and phase 3 clinical trials for the treatment of patients with T2DM showed similar efficacy and safety to alogliptin once daily and produced clinically and statistically significant improvements in glycaemic control with well tolerated in Japan [2, 3].
References:
[1]. McKeage, K., Trelagliptin: First Global Approval. Drugs, 2015. 75(10): p. 1161-4.
[2]. Inagaki, N., et al., Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol, 2015. 3(3): p. 191-7.
[3]. Inagaki, N., et al., SYR-472, a novel once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol, 2014. 2(2): p. 125-32.
- 2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile
Catalog No.:BCC8506
CAS No.:865758-96-9
- TC-E 5001
Catalog No.:BCC6355
CAS No.:865565-29-3
- Ganoderic acid SZ
Catalog No.:BCN4413
CAS No.:865543-37-9
- Junipediol A
Catalog No.:BCN6912
CAS No.:86548-91-6
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Bisisorhapontigenin A
Catalog No.:BCN3501
CAS No.:865474-98-2
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- apigenin 7-O-(6〃-O-malonyl)-β-D-glucoside
Catalog No.:BCN8399
CAS No.:86546-87-4
- Benazepril
Catalog No.:BCC4286
CAS No.:86541-75-5
- Benazepril HCl
Catalog No.:BCC5019
CAS No.:86541-74-4
- N-Methylcalycinine
Catalog No.:BCN4412
CAS No.:86537-66-8
- FR 180204
Catalog No.:BCC3669
CAS No.:865362-74-9
- PF-03084014
Catalog No.:BCC1848
CAS No.:865773-15-5
- Cytochrome c - pigeon (88-104)
Catalog No.:BCC1038
CAS No.:86579-06-8
- RX-3117
Catalog No.:BCC6381
CAS No.:865838-26-2
- Chamaejasmenin D
Catalog No.:BCN3046
CAS No.:865852-47-7
- Isochamaejasmenin B
Catalog No.:BCN3045
CAS No.:865852-48-8
- Tideglusib
Catalog No.:BCC4511
CAS No.:865854-05-3
- Oleuropeic acid 8-O-glucoside
Catalog No.:BCN4025
CAS No.:865887-46-3
- L-745,870 trihydrochloride
Catalog No.:BCC5695
CAS No.:866021-03-6
- 6-Benzoyl-5,7-dihydroxy-2,2-dimethylchromane
Catalog No.:BCN1326
CAS No.:86606-14-6
- Clausine Z
Catalog No.:BCN4414
CAS No.:866111-14-0
- PRX-08066
Catalog No.:BCC4209
CAS No.:866206-54-4
- PRX-08066 Maleic acid
Catalog No.:BCC1165
CAS No.:866206-55-5
Identification, characterization and HPLC quantification of process-related impurities in Trelagliptin succinate bulk drug: Six identified as new compounds.[Pubmed:27209451]
J Pharm Biomed Anal. 2016 Sep 5;128:18-27.
A sensitive, selective and stability indicating reversed-phase LC method was developed for the determination of process related impurities of Trelagliptin succinate in bulk drug. Six impurities were identified by LC-MS. Further, their structures were characterized and confirmed utilizing LC-MS/MS, IR and NMR spectral data. The most probable mechanisms for the formation of these impurities were also discussed. To the best of our knowledge, six structures among these impurities are new compounds and have not been reported previously. The superior separation was achieved on an InertSustain C18 (250mmx4.6mm, 5mum) column in a gradient mixture of acetonitrile and 20mmol potassium dihydrogen phosphate with 0.25% triethylamine (pH adjusted to 3.5 with phosphate acid). The method was validated as per regulatory guidelines to demonstrate system suitability, specificity, sensitivity, linearity, robustness, and stability.
A rapid and sensitive UHPLC-MS/MS assay for the determination of trelagliptin in rat plasma and its application to a pharmacokinetic study.[Pubmed:27561183]
J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Oct 15;1033-1034:166-171.
This study aims to develop and validate a simple, rapid and sensitive ultra-performance liquid chromatography with tandem mass spectrometry (UHPLC-MS/MS) method for exploring pharmacokinetic characteristics of Trelagliptin. Protein precipitation by acetonitrile was used to prepare plasma sample. A RRHD Eclipse Plus C18 (2.1x50mm, 1.8mu) column with gradient mobile phase (containing acetonitrile and 0.1% formic acid) help to achieve the separation of Trelagliptin and carbamazepine (IS) with high selectivity. Detection of target fragment ions m/z 358.2-->133.9 for Trelagliptin, and m/z 237.1-->194.0 for IS was performed in positive-ion electrospray ionization mass spectrometry by multiple reaction monitoring. Linear calibration plots were achieved in the range of 5-4000ng/mL for Trelagliptin (R(2)=0.999) in rat plasma. The recovery of Trelagliptin ranged from 87.8% to 93.7%. The method was showed to be accurate, precise and stable. No obvious matrix effect was found. It has been fully validated and successfully applied to pharmacokinetic study of Trelagliptin.
Trelagliptin (SYR-472, Zafatek), Novel Once-Weekly Treatment for Type 2 Diabetes, Inhibits Dipeptidyl Peptidase-4 (DPP-4) via a Non-Covalent Mechanism.[Pubmed:27328054]
PLoS One. 2016 Jun 21;11(6):e0157509.
Trelagliptin (SYR-472), a novel dipeptidyl peptidase-4 inhibitor, shows sustained efficacy by once-weekly dosing in type 2 diabetes patients. In this study, we characterized in vitro properties of Trelagliptin, which exhibited approximately 4- and 12-fold more potent inhibition against human dipeptidyl peptidase-4 than alogliptin and sitagliptin, respectively, and >10,000-fold selectivity over related proteases including dipeptidyl peptidase-8 and dipeptidyl peptidase-9. Kinetic analysis revealed reversible, competitive and slow-binding inhibition of dipeptidyl peptidase-4 by Trelagliptin (t1/2 for dissociation approximately 30 minutes). X-ray diffraction data indicated a non-covalent interaction between dipeptidyl peptidase and Trelagliptin. Taken together, potent dipeptidyl peptidase inhibition may partially contribute to sustained efficacy of Trelagliptin.
Effect of trelagliptin on vascular endothelial functions and serum adiponectin level in patients with type 2 diabetes: a preliminary single-arm prospective pilot study.[Pubmed:27809903]
Cardiovasc Diabetol. 2016 Nov 4;15(1):153.
BACKGROUND: Trelagliptin, an oral DPP-4 inhibitor, which is administered once per week and characterized by a long half-life in blood. The effects of Trelagliptin on vascular endothelial functions have not been clarified to date. The objective of the present study was to examine the effects of Trelagliptin on vascular endothelial functions in patients with type 2 diabetes mellitus (DM) using flow-mediated dilatation (FMD), adiponectin, and asymmetric dimethylarginine (ADMA) as evaluation indicators. METHODS: This study was a preliminary single-arm prospective pilot study. The subjects of this study were type 2 DM patients aged 20-74 years, who visited our outpatient department. The patients were treated with Trelagliptin, and their FMD, adiponectin, and ADMA levels were measured at baseline and at 12 weeks after initial treatment to determine the changes during the study period. RESULTS: A total of 27 patients, excluding three dropouts, were included in the population for analysis. Trelagliptin treatment showed no significant changes in FMD (2.42 +/- 2.7% at baseline vs. 2.66 +/- 3.8% post-treatment, P = 0.785) and ADMA (0.41 +/- 0.0 microg/mL at baseline vs. 0.40 +/- 0.0 microg/mL post-treatment, P = 0.402). Trelagliptin treatment resulted in a significant increase of serum adiponectin level (7.72 +/- 6.9 microg/mL at baseline vs. 8.82 +/- 8.3 microg/mL post-treatment, P < 0.002). CONCLUSIONS: In this pilot study, Trelagliptin treatment showed no significant changes in FMD. On the other hand, it was believed that Trelagliptin treatment may increase serum adiponectin level. Trial Registration http://www.umin.ac.jp (Trial ID UMIN000018311).


