FR 180204ERK inhibitor CAS# 865362-74-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 865362-74-9 | SDF | Download SDF |
| PubChem ID | 11493598 | Appearance | Powder |
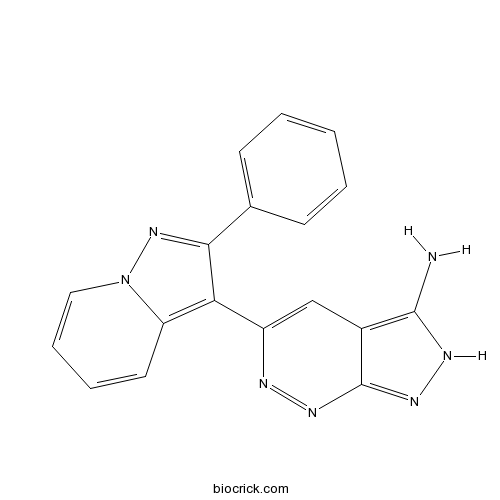
| Formula | C18H13N7 | M.Wt | 327.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (152.75 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-2H-pyrazolo[3,4-c]pyridazin-3-amine | ||
| SMILES | C1=CC=C(C=C1)C2=NN3C=CC=CC3=C2C4=CC5=C(NN=C5N=N4)N | ||
| Standard InChIKey | XVECMUKVOMUNLE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H13N7/c19-17-12-10-13(20-22-18(12)23-21-17)15-14-8-4-5-9-25(14)24-16(15)11-6-2-1-3-7-11/h1-10H,(H3,19,21,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective ERK inhibitor (IC50 values are 0.14 and 0.31 μM for ERK2 and ERK1 respectively). Displays 30-fold selectivity for ERK over p38α (IC50 = 10 μM); displays no activity against human recombinant MEK1, MKK4, IKKα, PKCα, Src, Syc and PDGFα at concentrations less than 30 μM. Also inhibits TGFβ-induced AP-1 activation in Mv1Lu cells (IC50 = 3.1 μM). |

FR 180204 Dilution Calculator

FR 180204 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0549 mL | 15.2746 mL | 30.5493 mL | 61.0986 mL | 76.3732 mL |
| 5 mM | 0.611 mL | 3.0549 mL | 6.1099 mL | 12.2197 mL | 15.2746 mL |
| 10 mM | 0.3055 mL | 1.5275 mL | 3.0549 mL | 6.1099 mL | 7.6373 mL |
| 50 mM | 0.0611 mL | 0.3055 mL | 0.611 mL | 1.222 mL | 1.5275 mL |
| 100 mM | 0.0305 mL | 0.1527 mL | 0.3055 mL | 0.611 mL | 0.7637 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selective ERK inhibitor (IC50 values are 0.14 and 0.31 μM for ERK2 and ERK1 respectively). Displays 30-fold selectivity for ERK over p38α (IC50 = 10 μM); displays no activity against human recombinant MEK1, MKK4, IKKα, PKCα, Src, Syc and PDGFα at concentrations less than 30 μM. Also inhibits TGFβ-induced AP-1 activation in Mv1Lu cells (IC50 = 3.1 μM).
- 4,5-dihydroxy-3,8-dimethylnaphthalene-1,2-dione
Catalog No.:BCN8422
CAS No.:86533-36-0
- AMG837
Catalog No.:BCC6387
CAS No.:865231-46-5
- Gelsempervine A
Catalog No.:BCN3929
CAS No.:865187-17-3
- Vinblastine
Catalog No.:BCN2376
CAS No.:865-21-4
- BMS-663068 Tris
Catalog No.:BCC1429
CAS No.:864953-39-9
- BMS-663068
Catalog No.:BCC1428
CAS No.:864953-29-7
- Leojaponin
Catalog No.:BCN7381
CAS No.:864817-63-0
- Resminostat (RAS2410)
Catalog No.:BCC2165
CAS No.:864814-88-0
- Gnetucleistol C
Catalog No.:BCN3395
CAS No.:864763-61-1
- Gnetucleistol B
Catalog No.:BCN3585
CAS No.:864763-60-0
- TC-MCH 7c
Catalog No.:BCC6149
CAS No.:864756-35-4
- SB 699551
Catalog No.:BCC7594
CAS No.:864741-95-7
- N-Methylcalycinine
Catalog No.:BCN4412
CAS No.:86537-66-8
- Benazepril HCl
Catalog No.:BCC5019
CAS No.:86541-74-4
- Benazepril
Catalog No.:BCC4286
CAS No.:86541-75-5
- apigenin 7-O-(6〃-O-malonyl)-β-D-glucoside
Catalog No.:BCN8399
CAS No.:86546-87-4
- Narlaprevir
Catalog No.:BCC1785
CAS No.:865466-24-6
- Bisisorhapontigenin A
Catalog No.:BCN3501
CAS No.:865474-98-2
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Junipediol A
Catalog No.:BCN6912
CAS No.:86548-91-6
- Ganoderic acid SZ
Catalog No.:BCN4413
CAS No.:865543-37-9
- TC-E 5001
Catalog No.:BCC6355
CAS No.:865565-29-3
- 2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile
Catalog No.:BCC8506
CAS No.:865758-96-9
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
TeA is a key virulence factor for Alternaria alternata (Fr.) Keissler infection of its host.[Pubmed:28324684]
Plant Physiol Biochem. 2017 Jun;115:73-82.
A toxin-deficient mutant strain, HP001 mutant of Alternaria alternata, whose mycelium is unable to infect its host, produces little tenuazonic acid (TeA) toxin. How TeA plays a role in initiating host infection by A. alternata remains unclear. In this research we use Imaging-PAM based on chlorophyll fluorescence parameters and transmission electron microscopy to explore the role of TeA toxin during the infection process of A. alternata. Photosystem II damage began even before wild type mycelium infected the leaves of its host, croftonweed (Ageratina adenophora). Compared with the wild type, HP001 mutant produces morphologically different colonies, hyphae with thinner cell walls, has higher reactive oxygen species (ROS) content and lower peroxidase activity, and fails to form appressoria on the host surface. Adding TeA toxin allows the mutant to partially recover these characters and more closely resemble the wild type. Additionally, we found that the mutant is able to elicit disease symptoms when its mycelium is placed on leaves whose epidermis has been manually removed, which indicates that TeA may be determinant in the fungus recognition of its plant host. Lack of TeA toxin appears responsible for the loss of pathogenicity of the HP001 mutant. As a key virulence factor, TeA toxin not only damages the host plant but also is involved in maintaining ROS content, host recognition, inducing appressoria to infect the host and for allowing completion of the infection process.
Vessel Patency at 24 Hours and Its Relationship With Clinical Outcomes and Infarct Volume in REVASCAT Trial (Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset).[Pubmed:28292867]
Stroke. 2017 Apr;48(4):983-989.
BACKGROUND AND PURPOSE: Higher rates of target vessel patency at 24 hours were noted in the thrombectomy group compared with control group in recent randomized trials. As a prespecified secondary end point, we aimed to assess 24-hour revascularization rates by treatment groups and occlusion site as they related to clinical outcome and 24-hour infarct volume in REVASCAT (Randomized Trial of Revascularization With Solitaire FR Device Versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset). METHODS: Independent core laboratory adjudicated vessel status according to modified arterial occlusive lesion classification at 24 hours on computed tomographic/magnetic resonance (94.2%/5.8%) angiography and 24-hour infarct volume on computed tomography were studied (95/103 patients in the thrombectomy group versus 94/103 in the control group, respectively). Complete revascularization was defined as modified arterial occlusive lesion grade 3. Its effect on clinical outcome was analyzed by ordinal logistic regression. RESULTS: Complete revascularization was achieved in 70.5% of the solitaire group and in 22.3% of the control group (P<0.001). Significant differences in complete revascularization rates were found for terminus internal carotid artery, M1, and tandem occlusions (all P<0.001) but not for M2 occlusions. In the thrombectomy group, 2 out of 63 patients (3.1%) with modified Thrombolysis in Cerebral Infarction 2b/3 after thrombectomy showed arterial reocclusion (modified arterial occlusive lesion grade 0/1) at 24 hours. Complete revascularization was associated with improved outcome in both thrombectomy (adjusted odds ratio, 4.5; 95% confidence interval, 1.9-10.9) and control groups (adjusted odds ratio, 2.7; 95% confidence interval, 1.0-6.7). Revascularization (modified arterial occlusive lesion grade 2/3) was associated with smaller infarct volumes in either treatment arm. CONCLUSIONS: Complete revascularization at 24 hours is a powerful predictor of favorable clinical outcome, whereas revascularization of any type results in reduced infarct volume in both thrombectomy and control groups. CLINICAL TRIAL REGISTRATION: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01692379.
The CAM-ICU has now a French "official" version. The translation process of the 2014 updated Complete Training Manual of the Confusion Assessment Method for the Intensive Care Unit in French (CAM-ICU.fr).[Pubmed:28365244]
Anaesth Crit Care Pain Med. 2017 Oct;36(5):297-300.
INTRODUCTION: Delirium is common in Intensive-Care-Unit (ICU) patients but under-recognized by bed-side clinicians when not using validated delirium-screening tools. The Confusion-Assessment-Method for the ICU (CAM-ICU) has demonstrated very good psychometric properties, and has been translated into many different languages though not into French. We undertook this opportunity to describe the translation process. MATERIAL AND METHODS: The translation was performed following recommended guidelines. The updated method published in 2014 including introduction letters, worksheet and flowsheet for bed-side use, the method itself, case-scenarios for training and Frequently-Asked-Questions (32 pages) was translated into French language by a neuropsychological researcher who was not familiar with the original method. Then, the whole method was back-translated by a native English-French bilingual speaker. The new English version was compared to the original one by the Vanderbilt University ICU-delirium-team. Discrepancies were discussed between the two teams before final approval of the French version. RESULTS: The entire process took one year. Among the 3692 words of the back-translated version of the method itself, 18 discrepancies occurred. Eight (44%) lead to changes in the final version. Details of the translation process are provided. CONCLUSIONS AND RELEVANCE: The French version of CAM-ICU is now available for French-speaking ICUs. The CAM-ICU is provided with its complete training-manual that was challenging to translate following recommended process. While many such translations have been done for other clinical tools, few have published the details of the process itself. We hope that the availability of such teaching material will now facilitate a large implementation of delirium-screening in French-speaking ICUs.
[Comparing study on the hyoid bone position after treatment of class malocclusion using improved appliance FR ].[Pubmed:28317354]
Hua Xi Kou Qiang Yi Xue Za Zhi. 2016 Aug 1;34(4):369-374.
OBJECTIVE: This study aims to compare the changes of hyoid bone position before and after treatment of Angle class malocclusion using improved appliance FR . METHODS: Forty patients with Angle class malocclusion were chosen and divided into two groups, namely, experimental and control. Each group had 20 patients. The young patients in the experi-mental group were treated using improved appliance FR , whereas those in the control group were treated using classic appliance FR . The hyoid bone position of the two groups were comparatively analyzed using an X-ray film before and after treatment. RESULTS: Compared with the condition before treatment, the condition after treatment showed that the hyoid bone position of young patients with Angle class malocclusion treated using improved appliance FR , H-FH, H-S, H-Ptm, and Ar-H-Me exhibited an increased angle (P<0.01), whereas the hyoid bone position of those treated using H-MP and H-Gn showed a decreased angle (P<0.01). The hyoid bone position of young patients with Angle class malocclusion treated using classic appliance FR , H-FH, H-S, and H-Ptm had an increased angle (P<0.05). Moreover, the hyoid bone position of those treated using Ar-H-Me had an increased angle (P<0.01), and the hyoid bone position of those treated using H-MP and H-RGn had a decreased angle (P<0.05). CONCLUSIONS: Compared with the hyoid bone position before treatment, the hyoid bone position after treatment of the young patients with Angle class malocclusion treated using improved appliance FR may move backward and downward, and the mandibular and hyoid bone position may move through clockwise rotation. The mandibular and hyoid bone position of young patients with Angle class malocclusion treated using classic appliance FR obtained a large angle by moving clockwise. The man-dibular bone moves backward and downward, thereby improving the hyoid bone in backward and upward directions. This condition makes a significant difference in treating the hyoid bone position of young patients with functional Angle class malocclusion..
Signaling to extracellular signal-regulated kinase from ErbB1 kinase and protein kinase C: feedback, heterogeneity, and gating.[Pubmed:23754287]
J Biol Chem. 2013 Jul 19;288(29):21001-14.
Many extracellular signals act via the Raf/MEK/ERK cascade in which kinetics, cell-cell variability, and sensitivity of the ERK response can all influence cell fate. Here we used automated microscopy to explore the effects of ERK-mediated negative feedback on these attributes in cells expressing endogenous ERK or ERK2-GFP reporters. We studied acute rather than chronic stimulation with either epidermal growth factor (ErbB1 activation) or phorbol 12,13-dibutyrate (PKC activation). In unstimulated cells, ERK-mediated negative feedback reduced the population-average and cell-cell variability of the level of activated ppERK and increased its robustness to changes in ERK expression. In stimulated cells, negative feedback (evident between 5 min and 4 h) also reduced average levels and variability of phosphorylated ERK (ppERK) without altering the "gradedness" or sensitivity of the response. Binning cells according to total ERK expression revealed, strikingly, that maximal ppERK responses initially occur at submaximal ERK levels and that this non-monotonic relationship changes to an increasing, monotonic one within 15 min. These phenomena occur in HeLa cells and MCF7 breast cancer cells and in the presence and absence of ERK-mediated negative feedback. They were best modeled assuming distributive (rather than processive) activation. Thus, we have uncovered a novel, time-dependent change in the relationship between total ERK and ppERK levels that persists without negative feedback. This change makes acute response kinetics dependent on ERK level and provides a "gating" or control mechanism in which the interplay between stimulus duration and the distribution of ERK expression across cells could modulate the proportion of cells that respond to stimulation.
Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex.[Pubmed:16139248]
Biochem Biophys Res Commun. 2005 Oct 14;336(1):357-63.
Selective inhibition of extracellular signal-regulated kinase (ERK) represents a potential approach for the treatment of cancer and other diseases; however, no selective inhibitors are currently available. Here, we describe an ERK-selective inhibitor, FR180204, and determine the structural basis of its selectivity. FR180204 inhibited the kinase activity of ERK1 and ERK2, with K(i) values 0.31 and 0.14microM, respectively. Lineweaver-Burk analysis of the binding interaction revealed that FR180204 acted as competitive inhibitor of ATP. In mink lung epithelial Mv1Lu cells, FR180204 inhibited TGFbeta-induced luciferase-expression. X-ray crystal structure analysis of the human ERK2/FR180204 complex revealed that Q105, D106, L156, and C166, which form the ATP-binding pocket on ERK, play important roles in the drug/protein interaction. These results suggest that FR180204 is an ERK-selective and cell-permeable inhibitor, and could be useful for elucidating the roles of ERK as well as for drug development.


