Tideglusibnon-ATP-competitive GSK-3β inhibitor CAS# 865854-05-3 |
- 5-OMe-UDP trisodium salt
Catalog No.:BCC6153
CAS No.:1207530-98-0
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
- Prasugrel hydrochloride
Catalog No.:BCC4291
CAS No.:389574-19-0
- Prasugrel Maleic acid
Catalog No.:BCC4292
CAS No.:389574-20-3
- MRS 2578
Catalog No.:BCC4976
CAS No.:711019-86-2
- AZD1283
Catalog No.:BCC5370
CAS No.:919351-41-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 865854-05-3 | SDF | Download SDF |
| PubChem ID | 11313622 | Appearance | Powder |
| Formula | C19H14N2O2S | M.Wt | 334.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NP-12; NP031112 | ||
| Solubility | DMSO : 33.33 mg/mL (99.67 mM; Need ultrasonic) | ||
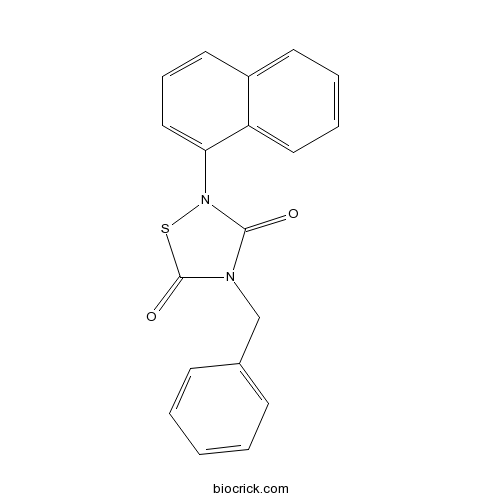
| Chemical Name | 4-benzyl-2-naphthalen-1-yl-1,2,4-thiadiazolidine-3,5-dione | ||
| SMILES | C1=CC=C(C=C1)CN2C(=O)N(SC2=O)C3=CC=CC4=CC=CC=C43 | ||
| Standard InChIKey | PMJIHLSCWIDGMD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H14N2O2S/c22-18-20(13-14-7-2-1-3-8-14)19(23)24-21(18)17-12-6-10-15-9-4-5-11-16(15)17/h1-12H,13H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tideglusib is an irreversible GSK-3 inhibitor with IC50 of 5 nM and 60 nM for GSK-3βWT (1 h preincubation) and GSK-3βC199A (1 h preincubation), respectively.In Vitro:Tideglusib (NP12) is a small heterocyclic thiadiazolidinone (TDZD) derivative, which is an ATP-non competitive inhibitor of GSK-3β with an IC50 value in the micromolar range[2]. Incubation of both astrocyte and microglial cultures with Tideglusib (NP031112) completely abrogates the induction of TNF-α and COX-2 expression after glutamate treatment. These effects of NP031112 are not caused by a loss of cell viability, because the 24 h exposure of astrocyte and microglial cells to this TDZD does not modify cell viability[3].In Vivo:Tideglusib (NP12) treatment correlates with an increase of 46% as an average in the inhibitory phosphorylation of GSK-3β at Ser-9 in the brains of APPsw-tauvlw mice, and the levels of the inactive from of the enzyme in NP12 treated mice are comparable to those found in wild-type littermate controls (p=0.893) (n=6-8 for each treatment). NP12 treatment results in significantly decreased phosphorylation at the putative GSK-3β-directed sites Ser-202 (CP13) and Ser-396/404 (PHF-1) in 15-month-old mice by more than 60% (p=0.023 and p=0.024, respectively)[2]. Injection of Tideglusib (NP031112) (50 mg/kg) into the rat hippocampus dramatically reduces kainic acid-induced inflammation, as measured by edema formation using T2-weighted magnetic resonance imaging and glial activation and has a neuroprotective effect in the damaged areas of the hippocampus[3]. References: | |||||

Tideglusib Dilution Calculator

Tideglusib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9905 mL | 14.9526 mL | 29.9052 mL | 59.8104 mL | 74.763 mL |
| 5 mM | 0.5981 mL | 2.9905 mL | 5.981 mL | 11.9621 mL | 14.9526 mL |
| 10 mM | 0.2991 mL | 1.4953 mL | 2.9905 mL | 5.981 mL | 7.4763 mL |
| 50 mM | 0.0598 mL | 0.2991 mL | 0.5981 mL | 1.1962 mL | 1.4953 mL |
| 100 mM | 0.0299 mL | 0.1495 mL | 0.2991 mL | 0.5981 mL | 0.7476 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: A potent, selective and irreversible non-ATP-competitive GSK-3β suppressor with an IC50 of 60 nM.
Tideglusib is a GSK-3 inhibitor currently undergoing phase II clinical trials for Alzheimer disease and progressive supranuclear palsy. Sustained oral administration of Tideglusib to animal models could down-regulates Tau hyper-phosphorylation, reduces brain amyloid plaque load, promotes learning and memory as well as prevents neuronal loss. [1]
In vitro: In vitro studies showed that after the unbound Tideglusib was removed from the reaction medium, the enzyme function could not be recovered. In addition, the dissociation rate constant of the reaction was as low as nearly zero. All above findings suggested that Tideglusib blocked GSK-3 irreversibly. Such irreversibility might be responsible for the non-competitive inhibition pattern with respect to ATP of Tideglusib and perhaps other structurally related compounds. [1]
In vivo: Based on double transgenic mice model co-expressing human mutant APP and tau, a study demonstrated that Tideglusib could suppress GSK-3, reduced amyloid and tau pathologies, blocked neuronal cell death and memory deficits in vivo. [2]
Clinical trial: A pilot, double-blind, placebo-controlled and randomized clinical trial was conducted to study the effect of Tideglusib in AD patients with an escalating dose. Thirty patients with mild to moderate AD were orally administered with Tideglusib in escalating doses of 400, 600, 800 and 1000 mg for periods of 4, 4, 6 and 6 weeks, respectively. This pilot study proved the safety and effectiveness of Tideglusib in AD patients. [3]
References:
[1]Domínguez JM, Fuertes A, Orozco L, Monte-Millan MD, Delgado E and Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3 by Tideglusib. J Biol Chem. 2012 Jan; 287(2): 893-904.
[2]Serenóa L, Coma M, Rodríguez M, Sánchez-Ferrer P, Sánchez MB, Gich I, Agulló JM, Pérez M, Avila J, Guardia-Laguarta C, Clarimón J, Lleó A, Gómez-Isla T. A novel GSK-3β inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009 Sep; 35(3): 359-67.
[3]del Ser, T. Phase IIA clinical trial on Alzheimer’s Disease with NP-12, a GSK-3 inhibitor. Alzheimers and Dement. 2010; 6: S147.
- Isochamaejasmenin B
Catalog No.:BCN3045
CAS No.:865852-48-8
- Chamaejasmenin D
Catalog No.:BCN3046
CAS No.:865852-47-7
- RX-3117
Catalog No.:BCC6381
CAS No.:865838-26-2
- Cytochrome c - pigeon (88-104)
Catalog No.:BCC1038
CAS No.:86579-06-8
- PF-03084014
Catalog No.:BCC1848
CAS No.:865773-15-5
- Trelagliptin
Catalog No.:BCC2014
CAS No.:865759-25-7
- 2-[(6-Chloro-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl)methyl]benzonitrile
Catalog No.:BCC8506
CAS No.:865758-96-9
- TC-E 5001
Catalog No.:BCC6355
CAS No.:865565-29-3
- Ganoderic acid SZ
Catalog No.:BCN4413
CAS No.:865543-37-9
- Junipediol A
Catalog No.:BCN6912
CAS No.:86548-91-6
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Bisisorhapontigenin A
Catalog No.:BCN3501
CAS No.:865474-98-2
- Oleuropeic acid 8-O-glucoside
Catalog No.:BCN4025
CAS No.:865887-46-3
- L-745,870 trihydrochloride
Catalog No.:BCC5695
CAS No.:866021-03-6
- 6-Benzoyl-5,7-dihydroxy-2,2-dimethylchromane
Catalog No.:BCN1326
CAS No.:86606-14-6
- Clausine Z
Catalog No.:BCN4414
CAS No.:866111-14-0
- PRX-08066
Catalog No.:BCC4209
CAS No.:866206-54-4
- PRX-08066 Maleic acid
Catalog No.:BCC1165
CAS No.:866206-55-5
- 3,4,5-Tricaffeoylquinic acid
Catalog No.:BCN2384
CAS No.:86632-03-3
- Xanthiside
Catalog No.:BCN2545
CAS No.:866366-86-1
- 7-Ethyl-10-Hydroxycamptothecin
Catalog No.:BCN2479
CAS No.:86639-52-3
- 10-Aminocamptothecin
Catalog No.:BCC8111
CAS No.:86639-63-6
- DPPI 1c hydrochloride
Catalog No.:BCC2363
CAS No.:866396-34-1
- Dorsomorphin
Catalog No.:BCC5131
CAS No.:866405-64-3
A Four-Point Screening Method for Assessing Molecular Mechanism of Action (MMOA) Identifies Tideglusib as a Time-Dependent Inhibitor of Trypanosoma brucei GSK3beta.[Pubmed:26942720]
PLoS Negl Trop Dis. 2016 Mar 4;10(3):e0004506.
BACKGROUND: New therapeutics are needed for neglected tropical diseases including Human African trypanosomiasis (HAT), a progressive and fatal disease caused by the protozoan parasites Trypanosoma brucei gambiense and T. b. rhodesiense. There is a need for simple, efficient, cost effective methods to identify new molecules with unique molecular mechanisms of action (MMOAs). The mechanistic features of a binding mode, such as competition with endogenous substrates and time-dependence can affect the observed inhibitory IC50, and differentiate molecules and their therapeutic usefulness. Simple screening methods to determine time-dependence and competition can be used to differentiate compounds with different MMOAs in order to identify new therapeutic opportunities. METHODOLOGY/PRINCIPAL FINDINGS: In this work we report a four point screening methodology to evaluate the time-dependence and competition for inhibition of GSK3beta protein kinase isolated from T. brucei. Using this method, we identified Tideglusib as a time-dependent inhibitor whose mechanism of action is time-dependent, ATP competitive upon initial binding, which transitions to ATP non-competitive with time. The enzyme activity was not recovered following 100-fold dilution of the buffer consistent with an irreversible mechanism of action. This is in contrast to the T. brucei GSK3beta inhibitor GW8510, whose inhibition was competitive with ATP, not time-dependent at all measured time points and reversible in dilution experiments. The activity of Tideglusib against T. brucei parasites was confirmed by inhibition of parasite proliferation (GI50 of 2.3 muM). CONCLUSIONS/SIGNIFICANCE: Altogether this work demonstrates a straightforward method for determining molecular mechanisms of action and its application for mechanistic differentiation of two potent TbGSK3beta inhibitors. The four point MMOA method identified Tideglusib as a mechanistically differentiated TbGSK3beta inhibitor. Tideglusib was shown to inhibit parasite growth in this work, and has been reported to be well tolerated in one year of dosing in human clinical studies. Consequently, further supportive studies on the potential therapeutic usefulness of Tideglusib for HAT are justified.
Tideglusib induces apoptosis in human neuroblastoma IMR32 cells, provoking sub-G0/G1 accumulation and ROS generation.[Pubmed:27490211]
Environ Toxicol Pharmacol. 2016 Sep;46:194-205.
Neuroblastoma is the most common tumor amongst children amounting to nearly 15% of cancer deaths. This cancer is peculiar in its characteristics, exhibiting differentiation, maturation and metastatic transformation leading to poor prognosis and low survival rates among children. Chemotherapy, though toxic to normal cells, has shown to improve the survival of the patient with emphasis given more towards targeting angiogenesis. Recently, Tideglusib was designed as an 'Orphan Drug' to target the neurodegenerative Alzheimer's disease and gained significant momentum in its function during clinical trials. Duffy et al. recently reported a reduction in cell viability of human IMR32 neuroblastoma cells when treated with Tideglusib at varying concentrations. We investigated the effects of Tideglusib, at various concentrations, compared to Lithium chloride at various concentrations, on IMR32 cells. Lithium, a known GSK-3 inhibitor, was used as a standard to compare the efficiency of Tideglusib in a dose-dependent manner. Cell viability was assessed by MTT assay. The stages of apoptosis were evaluated by AO/EB staining and nuclear damage was determined by Hoechst 33258 staining. Reactive oxygen species (ROS) and mitochondrial membrane potential (DeltaPsim) were assessed by DCFDA dye and Rhodamine-123 dye, respectively. Tideglusib reported a significant dose-dependent increase in pro-apoptotic proteins (PARP, Caspase-9, Caspase-7, Caspase-3) and tumor-related genes (FasL, TNF-alpha, Cox-2, IL-8, Caspase-3). Anti-GSK3 beta, pGSK3 beta, Bcl-2, Akt-1, p-Akt1 protein levels were observed with cells exposed to Tideglusib and Lithium chloride. No significant dose-dependent changes were observed for the mRNA expression of collagenase MMP-2, the tumor suppressor p53, or the cell cycle protein p21. Our study also reports Tideglusib reducing colony formation and increasing the level of sub-G0/G1 population in IMR32 cells. Our investigations report the significance of Tideglusib as a promising apoptotic inducer in human neuroblastoma IMR32 cells. Our study also reports that LiCl reduced cell viability in IMR32 cells inducing apoptosis mediated by ROS generation.
Tideglusib, a chemical inhibitor of GSK3beta, attenuates hypoxic-ischemic brain injury in neonatal mice.[Pubmed:27378458]
Biochim Biophys Acta. 2016 Oct;1860(10):2076-85.
BACKGROUND: Hypoxia-ischemia is an important cause of brain injury and neurological morbidity in the newborn infants. The activity of glycogen synthase kinase-3beta (GSK-3beta) is up-regulated following neonatal stroke. Tideglusib is a GSK-3beta inhibitor which has neuroprotective effects against neurodegenerative diseases in clinical trials. However, the effect of Tideglusib on hypoxic-ischemic (HI) brain injury in neonates is still unknown. METHODS: Postnatal day 7 (P7) mouse pups subjected to unilateral common carotid artery ligation followed by 1h of hypoxia or sham surgery was performed. HI animals were administered Tideglusib (5mg/kg) or vehicle intraperitoneally 20min prior to the onset of ischemia. The brain infarct volume and whole brain images, were used in conjunction with Nissl staining to evaluate the protective effects of Tideglusib. Protein levels of glial fibrillary acidic protein (GFAP), Notch1, cleaved caspase-3/9, phosphorylated signal transducer and activator of transcription 3 (STAT3), GSK-3beta and protein kinase B (Akt) were detected to identify potentially involved molecules. RESULTS: Tideglusib significantly reduced cerebral infarct volume at both 24h and 7days after HI injury. Tideglusib also increased phosphorylated GSK-3beta(Ser9) and Akt(Ser473), and reduced the expression of GFAP and p-STAT3(Tyr705). In addition, pretreatment with Tideglusib also enhanced the protein level of Notch1. Moreover, Tideglusib reduced the cleavage of pro-apoptotic signal caspase proteins, including caspase 3 and caspase 9 following HI. CONCLUSION: These results indicate that Tideglusib shows neuroprotection against hypoxic-ischemic brain injury in neonatal mice. GENERAL SIGNIFICANCE: Tideglusib is a potential compound for the prevention or treatment of hypoxic-ischemic brain injury in neonates.
Tideglusib protects neural stem cells against NMDA receptor overactivation.[Pubmed:26398371]
Pharmacol Rep. 2015 Oct;67(5):823-31.
BACKGROUND: N-methyl-d-aspartate (NMDA) receptors are major pharmacological targets to prevent or reduce the progression of neurodegenerative diseases. Successful therapy with NMDA receptor antagonists in humans has been limited by the severe side effects of complete receptor blockade. The aim of the present study was to investigate the possible protective effects of Tideglusib against NMDA receptor overactivation in neural stem cells. METHODS: We measured the alteration in membrane integrity, free radical generation, intracellular Ca(2+) accumulation, mitochondrial membrane potential (MMP)/mitochondrial morphology and glycogen synthase kinase-3 (alpha/beta isoforms and phospho-GSK-3alpha/beta) protein expression levels following treatments. RESULTS: NMDA treatment, with or without d-serine, significantly increased LDH leakage and triggered cell death in neural stem cells. Reactive oxygen species (ROS) formation and intracellular Ca(2+) levels were increased following NMDA receptor overactivation. The significant reduction in MMP was found in NMDA/d-serine-treated cells. Tideglusib significantly decreased ROS production and membrane degradation, but did not change intracellular Ca(2+) levels following NMDA receptor activation. Both in the presence or in the absence of NMDA/d-serine, Tideglusib increased MMP and the levels of phospho-GSK-3beta in NSCs. Moreover, GW9662 (a peroxisome-proliferator-activated receptor gamma (PPARgamma) antagonist) treatment significantly inhibited the protective effect of Tideglusib in NMDA/d-serine-treated cells. CONCLUSION: Our study provides the evidence that GSK-3beta and PPARgamma may be directly involved in pathways leading to NMDA receptor-induced cell death and that the inhibitors including Tideglusib may exert neuroprotective effect against these receptor overactivation.


