AR-C 66096 tetrasodium saltP2Y12 antagonist,potent and selective CAS# 145782-74-7 |
- TDZD-8
Catalog No.:BCC4258
CAS No.:327036-89-5
- TWS119
Catalog No.:BCC4512
CAS No.:601514-19-6
- GSK-3 inhibitor 1
Catalog No.:BCC4126
CAS No.:603272-51-1
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
- 1-Azakenpaullone
Catalog No.:BCC5332
CAS No.:676596-65-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145782-74-7 | SDF | Download SDF |
| PubChem ID | 90488830 | Appearance | Powder |
| Formula | C14H18F2N5Na4O12P3S | M.Wt | 703.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | FPL 66096, ARL 66096 | ||
| Solubility | Soluble in water (supplied pre-dissolved at a concentration of 10mM) | ||
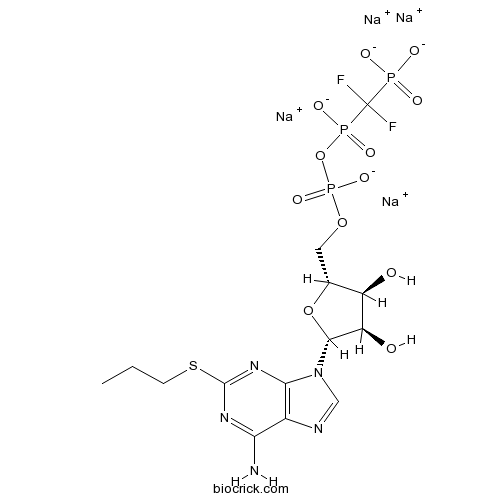
| Chemical Name | tetrasodium;[[(2R,3S,4R,5R)-5-(6-amino-2-propylsulfanylpurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl]oxy-[difluoro(phosphonato)methyl]phosphinate | ||
| SMILES | CCCSC1=NC2=C(C(=N1)N)N=CN2C3C(C(C(O3)COP(=O)([O-])OP(=O)(C(F)(F)P(=O)([O-])[O-])[O-])O)O.[Na+].[Na+].[Na+].[Na+] | ||
| Standard InChIKey | IETVOLFQZIGNAQ-HVYRMSERSA-J | ||
| Standard InChI | InChI=1S/C14H22F2N5O12P3S.4Na/c1-2-3-37-13-19-10(17)7-11(20-13)21(5-18-7)12-9(23)8(22)6(32-12)4-31-36(29,30)33-35(27,28)14(15,16)34(24,25)26;;;;/h5-6,8-9,12,22-23H,2-4H2,1H3,(H,27,28)(H,29,30)(H2,17,19,20)(H2,24,25,26);;;;/q;4*+1/p-4/t6-,8-,9-,12-;;;;/m1..../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective P2Y12 receptor antagonist. Blocks ADP-induced inhibition of adenylyl cyclase in vitro (pKB =7.6) and inhibits ADP-induced aggregation of washed human platelets (pIC50 = 8.16). |

AR-C 66096 tetrasodium salt Dilution Calculator

AR-C 66096 tetrasodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4219 mL | 7.1097 mL | 14.2195 mL | 28.439 mL | 35.5487 mL |
| 5 mM | 0.2844 mL | 1.4219 mL | 2.8439 mL | 5.6878 mL | 7.1097 mL |
| 10 mM | 0.1422 mL | 0.711 mL | 1.4219 mL | 2.8439 mL | 3.5549 mL |
| 50 mM | 0.0284 mL | 0.1422 mL | 0.2844 mL | 0.5688 mL | 0.711 mL |
| 100 mM | 0.0142 mL | 0.0711 mL | 0.1422 mL | 0.2844 mL | 0.3555 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
The platelet P2T receptor plays a major role in platelet aggregation, and its antagonists are suggested to have significant therapeutic potential as antithrombotic agents. AR-C 66096 is a novel, potent and selective antagonist at human platelet P2T-purinoceptors.
In vitro: In suspensions of human washed platelets, AR-C 66096 (1-100 nM) produced concentrationdependent rightward displacement of concentration-effect (E/[A]) curves obtained for ADP-induced platelet aggregation. The anti-aggregatory potency of AR-C 66096 was not influenced by increasing the incubation time from 2 to 15 min nor by inclusion of the P1-purinoceptor antagonist 8-sulphophenyltheophylline at a concentration (300 μM) [1].
In vivo: AR-C 66096 behaved as a weak (pA50 3.68) but full P2x-purinoceptor agonist in preparations of the isolated rabbit ear artery and as a weak, competitive antagonist (apparent pKB 4.71) at P2Y-purinoceptors in the isolated guinea-pig aorta, indicating a selectivity of at least 9000 fold for the Pzrsubtype. In the latter preparation, non-specific relaxations were observed by concentrations of AR-C 66096 >10 μM [1].
Clinical trial: Up to now, AR-C 66096 is still in the preclinical development stage.
Reference:
[1] Humphries, R. G.; Tomlinson, W.; Ingall, A. H.; Cage, P. A.; Leff, P. FPL 66096: A Novel, Highly Potent and Selective Antagonist at Human Platelet P2T-purinoceptors. Br. J. Pharmacol. 1994, 113, 1057-1063.
- 3-Allylrhodanine
Catalog No.:BCC8604
CAS No.:1457-47-2
- HG-9-91-01
Catalog No.:BCC4071
CAS No.:1456858-58-4
- SH-4-54
Catalog No.:BCC5483
CAS No.:1456632-40-8
- 2'-O-Benzoylpaeoniflorin
Catalog No.:BCN7803
CAS No.:1456598-64-3
- NNC 711
Catalog No.:BCC7176
CAS No.:145645-62-1
- Cyclocommunol
Catalog No.:BCN3375
CAS No.:145643-96-5
- Sophocarpine
Catalog No.:BCN5971
CAS No.:145572-44-7
- Eucamalol
Catalog No.:BCN1648
CAS No.:145544-91-8
- Mitiglinide Calcium
Catalog No.:BCC5000
CAS No.:145525-41-3
- 1,7-Dimethoxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7539
CAS No.:145523-71-3
- (2R,3S)-Boc-3-Phenylisoserine
Catalog No.:BCN8362
CAS No.:145514-62-1
- MRS 2179 tetrasodium salt
Catalog No.:BCC5685
CAS No.:1454889-37-2
- 4,6-Dichloro-5-nitro-2-propylthiopyrimidine
Catalog No.:BCC8667
CAS No.:145783-14-8
- 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine
Catalog No.:BCC8666
CAS No.:145783-15-9
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- Margatoxin
Catalog No.:BCC7709
CAS No.:145808-47-5
- Tiagabine hydrochloride
Catalog No.:BCC5217
CAS No.:145821-59-6
- D-myo-Inositol-1,3,4,5-tetrakisphosphate, octapotassium salt
Catalog No.:BCC7058
CAS No.:145843-69-2
- Brachynoside
Catalog No.:BCN3749
CAS No.:145898-87-9
- CGP 52411
Catalog No.:BCC7667
CAS No.:145915-58-8
- CGP 53353
Catalog No.:BCC7363
CAS No.:145915-60-2
- 7,8,9,9-Tetradehydroisolariciresinol
Catalog No.:BCN1649
CAS No.:145918-59-8
- 3-Amino-3-phenyl-1-propanol
Catalog No.:BCC8608
CAS No.:14593-04-5
- 2-(Methylamino)ethylphosphonic acid
Catalog No.:BCN1763
CAS No.:14596-55-5
Activity of adenosine diphosphates and triphosphates on a P2Y(T) -type receptor in brain capillary endothelial cells.[Pubmed:11156575]
Br J Pharmacol. 2001 Jan;132(1):173-82.
1. A P2Y (nucleotide) receptor activity in a clonal population (B10) of rat brain capillary endothelial cells is coupled to inhibition of adenylyl cyclase and has functional similarities to the P2Y(T) (previously designated 'P2T') receptor for ADP of blood platelets. However, the only P2Y receptor which was detectable in a previous study of B10 cells by mRNA analysis was the P2Y(1) receptor, which elsewhere shows no transduction via cyclic nucleotides. We have sought here to clarify these issues. 2. The inhibition of forskolin-stimulated adenylyl cyclase induced by purified nucleotides was measured on B10 cells. The EC(50) value for 2-methylthioADP (2-MeSADP) was 2.2 nM and, surprisingly, 2-MeSATP was an almost equally strong agonist (EC(50)=3.5 nM). ATP and 2-ClATP were weak partial agonists (EC(50)=26 microM and 10 microM respectively) and under appropriate conditions could antagonise the activity on 2-MeSADP. 3. A known selective antagonist of the platelet P2Y(T) receptor, 2-propylthioadenosine-5'-(beta,gamma)-difluoromethylene) triphosphonate (AR-C 66096), was a competitive antagonist of this B10 cell receptor, with pK(B)=7.6. That ligand is inactive at the P2Y(1) receptor in the same cells. Conversely, the competitive P2Y(1) receptor antagonists, the 3', 5'- and 2', 5'-adenosine bis-monophosphates, are, instead, weak agonists at the adenylyl cyclase-inhibitory receptor. 4. The inhibition of adenylyl cyclase by 2-MeSADP was completely abolished by pertussis toxin. 5. In summary, these brain endothelial cells possess a P2Y(T)-type receptor in addition to the P2Y(1) receptor. The two have similarities in agonist profiles but are clearly distinguishable by antagonists and by their second messenger activations. The possible relationships between the B10 and platelet P2Y(T) receptors are discussed.
Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy.[Pubmed:9925726]
J Med Chem. 1999 Jan 28;42(2):213-20.
The platelet P2T receptor plays a major role in platelet aggregation, and its antagonists are predicted to have significant therapeutic potential as antithrombotic agents. We have explored analogues of adenosine triphosphate (ATP), which is a weak, nonselective but competitive P2T receptor antagonist. Modification of the polyphosphate side chain to prevent breakdown to the agonist adenosine diphosphate (ADP) and substitution of the adenine moiety to enhance affinity and selectivity for the P2T receptor led to the identification of 10e (AR-C67085MX), having an IC50 of 2.5 nM against ADP-induced aggregation of human platelets. Compound 10e was the first very potent antagonist of the P2T receptor, with a selectivity for that subtype of the P2 receptor family of >1000-fold. Further modification of the structure produced compound 10l (AR-C69931MX) having an IC50 of 0.4 nM. In vivo, at maximally effective antithrombotic doses, there is little prolongation of bleeding time (1.4-fold), which is in marked contrast to the 5-6-fold found with GPIIb/IIIa antagonists.
FPL 66096: a novel, highly potent and selective antagonist at human platelet P2T-purinoceptors.[Pubmed:7858849]
Br J Pharmacol. 1994 Nov;113(3):1057-63.
1. ADP-dependent platelet aggregation is mediated by the P2T-purinoceptor and is specifically inhibited by ATP, which is a competitive P2T-purinoceptor antagonist. However, ATP functions as an agonist at other P2-purinoceptor subtypes in other tissues and is, therefore, non-selective. This paper describes the effects of the novel ATP analogue, FPL 66096 (2-propylthio-D-beta,gamma-difluoromethylene ATP), on ADP-induced and ADP-independent aggregation of human washed platelets and in standard preparations containing P2X- (rabbit ear artery) and P2Y-purinoceptors (guinea-pig aorta). 2. In suspensions of human washed platelets, FPL 66096 (1-100 nM) produced concentration-dependent rightward displacement of concentration-effect (E/[A]) curves obtained for ADP-induced platelet aggregation. Logistic fitting of E/[A] data indicated that the effect of FPL 66096 was consistent with simple competition with a pKB value of 8.66. FPL 66096 (10-1000 nM) had no effect on aggregation produced by the thromboxane A2-mimetic, U46619 (0.1-10 microM) when the response to this agent was rendered ADP-independent by inclusion of the non-selective P2-purinoceptor antagonist, suramin (100 microM). 3. The anti-aggregatory potency of FPL 66096 was not influenced by increasing the incubation time from 2 to 15 min nor by inclusion of the P1-purinoceptor antagonist 8-sulphophenyltheophylline at a concentration (300 microM) that produced a 68 fold rightward displacement of the anti-aggregatory E/[A] curve for the P1-purinoceptor agonist, 5'-N-ethylcarboxamidoadenosine (0.1-1000 microM). 4. FLP 66096 behaved as a weak (pA" 3.68) but full P2x-purinoceptor agonist in preparations of the rabbit isolated ear artery and as a weak, competitive antagonist (apparent pKB 4.71) at P2Y purinoceptors in the guinea-pig isolated aorta, indicating a selectivity of at least 9000 fold for the P2t-subtype. In the latter preparation, non-specific relaxations were produced by concentrations of FPL 66096 >10M gM.5. These results indicate that FPL 66096 is a P2-purinoceptor antagonist of unprecedented potency and selectivity and that its effects are consistent with simple competition at the P2-purinoceptor. Therefore,FPL 66096 represents a novel pharmacological tool in the classification of P2-purinoceptors and in the elucidation of the mechanisms involved in activation of platelets by ADP.


