CGP 52411CAS# 145915-58-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145915-58-8 | SDF | Download SDF |
| PubChem ID | 1697 | Appearance | Powder |
| Formula | C20H15N3O2 | M.Wt | 329.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DAPH | ||
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
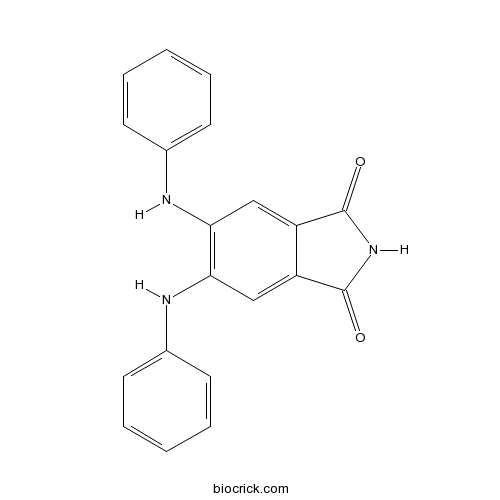
| Chemical Name | 5,6-dianilinoisoindole-1,3-dione | ||
| SMILES | C1=CC=C(C=C1)NC2=C(C=C3C(=C2)C(=O)NC3=O)NC4=CC=CC=C4 | ||
| Standard InChIKey | AAALVYBICLMAMA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H15N3O2/c24-19-15-11-17(21-13-7-3-1-4-8-13)18(12-16(15)20(25)23-19)22-14-9-5-2-6-10-14/h1-12,21-22H,(H,23,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of the epidermal growth factor receptor (EGFR) (IC50 = 0.3 μM in vitro). Also inhibits and reverses the formation of Aβ42 fibers associated with Alzheimer's disease. Eliminates calcium influx potential when coincubated with amyloid β-peptide (1-42); reduces neurotoxicity by blocking Ca2+ influx into neuronal cells. |

CGP 52411 Dilution Calculator

CGP 52411 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0363 mL | 15.1814 mL | 30.3628 mL | 60.7257 mL | 75.9071 mL |
| 5 mM | 0.6073 mL | 3.0363 mL | 6.0726 mL | 12.1451 mL | 15.1814 mL |
| 10 mM | 0.3036 mL | 1.5181 mL | 3.0363 mL | 6.0726 mL | 7.5907 mL |
| 50 mM | 0.0607 mL | 0.3036 mL | 0.6073 mL | 1.2145 mL | 1.5181 mL |
| 100 mM | 0.0304 mL | 0.1518 mL | 0.3036 mL | 0.6073 mL | 0.7591 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Brachynoside
Catalog No.:BCN3749
CAS No.:145898-87-9
- D-myo-Inositol-1,3,4,5-tetrakisphosphate, octapotassium salt
Catalog No.:BCC7058
CAS No.:145843-69-2
- Tiagabine hydrochloride
Catalog No.:BCC5217
CAS No.:145821-59-6
- Margatoxin
Catalog No.:BCC7709
CAS No.:145808-47-5
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine
Catalog No.:BCC8666
CAS No.:145783-15-9
- 4,6-Dichloro-5-nitro-2-propylthiopyrimidine
Catalog No.:BCC8667
CAS No.:145783-14-8
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
- 3-Allylrhodanine
Catalog No.:BCC8604
CAS No.:1457-47-2
- HG-9-91-01
Catalog No.:BCC4071
CAS No.:1456858-58-4
- SH-4-54
Catalog No.:BCC5483
CAS No.:1456632-40-8
- 2'-O-Benzoylpaeoniflorin
Catalog No.:BCN7803
CAS No.:1456598-64-3
- CGP 53353
Catalog No.:BCC7363
CAS No.:145915-60-2
- 7,8,9,9-Tetradehydroisolariciresinol
Catalog No.:BCN1649
CAS No.:145918-59-8
- 3-Amino-3-phenyl-1-propanol
Catalog No.:BCC8608
CAS No.:14593-04-5
- 2-(Methylamino)ethylphosphonic acid
Catalog No.:BCN1763
CAS No.:14596-55-5
- 2-Dimethylaminoethylphosphonic acid
Catalog No.:BCN1764
CAS No.:14596-56-6
- N,N,N-Trimethyl-2-aminoethylphosphonate
Catalog No.:BCN1560
CAS No.:14596-57-7
- Laccaic acid E
Catalog No.:BCN1807
CAS No.:14597-16-1
- Jasminoid A
Catalog No.:BCN7605
CAS No.:1459784-57-6
- Cycloart-23-ene-3,25-diol
Catalog No.:BCN2640
CAS No.:14599-48-5
- Yohimbine
Catalog No.:BCN2293
CAS No.:146-48-5
- 2-Chloroadenosine
Catalog No.:BCC7575
CAS No.:146-77-0
- 2-Fluoroadenosine
Catalog No.:BCC8576
CAS No.:146-78-1
Retinoic acid-induced RB (retinoblastoma) hypophosphorylation enhanced by CGP 52411 (4,5-dianilinophthalimide), an EGF family tyrosine kinase receptor inhibitor.[Pubmed:8741214]
Eur J Cell Biol. 1996 Apr;69(4):327-34.
Retinoic acid is a known morphogen which can regulate cell proliferation and differentiation and also induces the hypophosphorylation of the RB (retinoblastoma tumor suppressor gene) protein, a known cell cycle regulatory protein. The mechanism by which these processes occur is unclear. We find that these processes can be regulated by CGP 52411, 4,5-dianilinophthalimide, an inhibitor of tyrosine protein kinases of the EGF receptor subfamily. Retinoic acid causes the largely phosphorylated RB protein expressed in proliferating HL-60 human promyelocytic leukemia cells to shift to the unphosphorylated form, as well as causing the cells to G0 arrest and differentiate. Addition of CGP 52411 accelerated the redistribution of the RB protein expressed in HL-60 cells to the unphosphorylated form, enhancing the effects of the retinoic acid. By itself CGP 52411 had no apparent effect on the RB protein expressed in HL-60 cells. CGP 52411 also accelerated the retinoic acid-induced accumulation of cells in G1/0 and the phenotypic conversion of cells to the mature myeloid phenotype, suggesting that its target is common to the regulation of both RB phosphorylation and cell proliferation and differentiation. CGP 52411 had a similar effect on the RB phosphorylation shift induced by 1,25-dihydroxy vitamin D3, a ligand for a receptor in the same steroid thyroid hormone superfamily as retinoic acid. Increasing the concentration of CGP 52411 enhanced the acceleration of RB hypophosphorylation in the case of both retinoic acid and 1,25-dihydroxy vitamin D3. The data are consistent with the negative regulation of retinoic acid induced RB protein dephosphorylation coupled to cell cycle arrest and differentiation by a receptor tyrosine kinase sensitive to CGP 52411.
Modelling study of protein kinase inhibitors: binding mode of staurosporine and origin of the selectivity of CGP 52411.[Pubmed:8789188]
J Comput Aided Mol Des. 1995 Dec;9(6):465-72.
A model for the binding mode of the potent protein kinase inhibitor staurosporine is proposed. Using the information provided by the crystal structure of the cyclic-AMP-dependent protein kinase, it is suggested that staurosporine, despite a seemingly unrelated chemical structure, exploits the same key hydrogen-bond interactions as ATP, the cofactor of the protein kinases, in its binding mode. The structure-activity relationship of the inhibitor and a docking analysis give strong support to this hypothesis. The selectivity of the dianilinophthalimide inhibitor CGP 52411 towards the EGF-receptor protein tyrosine kinase is rationalized on the basis of the model. It is proposed that this selectivity originates in the occupancy, by one of the anilino moieties of the inhibitor, of the region of the enzyme cleft that normally binds the ribose ring of ATP, which appears to possess a marked lipophilic character in this kinase.
Direct and selective elimination of specific prions and amyloids by 4,5-dianilinophthalimide and analogs.[Pubmed:18480256]
Proc Natl Acad Sci U S A. 2008 May 20;105(20):7159-64.
Mechanisms to safely eliminate amyloids and preamyloid oligomers associated with many devastating diseases are urgently needed. Biophysical principles dictate that small molecules are unlikely to perturb large intermolecular protein-protein interfaces, let alone extraordinarily stable amyloid interfaces. Yet 4,5-dianilinophthalimide (DAPH-1) reverses Abeta42 amyloidogenesis and neurotoxicity, which is associated with Alzheimer's disease. Here, we show that DAPH-1 and select derivatives are ineffective against several amyloidogenic proteins, including tau, alpha-synuclein, Ure2, and PrP, but antagonize the yeast prion protein, Sup35, in vitro and in vivo. This allowed us to exploit several powerful new tools created for studying the conformational transitions of Sup35 and decipher the mechanisms by which DAPH-1 and related compounds antagonize the prion state. During fibrillization, inhibitory DAPHs alter the folding of Sup35's amyloidogenic core, preventing amyloidogenic oligomerization and specific recognition events that nucleate prion assembly. Select DAPHs also are capable of attacking preformed amyloids. They remodel Sup35 prion-specific intermolecular interfaces to create morphologically altered aggregates with diminished infectivity and self-templating activity. Our studies provide mechanistic insights and reinvigorate hopes for small-molecule therapies that specifically disrupt intermolecular amyloid contacts.
Efficient reversal of Alzheimer's disease fibril formation and elimination of neurotoxicity by a small molecule.[Pubmed:15388848]
Proc Natl Acad Sci U S A. 2004 Oct 5;101(40):14326-32.
The Abeta1-42 peptide that is overproduced in Alzheimer's disease (AD) from a large precursor protein has a normal amino acid sequence but, when liberated, misfolds at neutral pH to form "protofibrils" and fibrils that are rich in beta-sheets. We find that these protofibrils or fibrils are toxic to certain neuronal cells that carry Ca-permeant alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Disrupting the structure of the Abeta1-42 fibrils and protofibrils might lead to the discovery of molecules that would be very useful in the treatment of AD. A high-throughput screen of a library of >3,000 small molecules with known "biological activity" was set up to find compounds that efficiently decrease the beta-sheet content of aggregating Abeta1-42. Lead compounds were characterized by using thioflavin T (ThT) as a beta-sheet assay. The most effective of six compounds found was 4,5-dianilinophthalimide (DAPH) under the following conditions: DAPH at low micromolar concentrations abolishes or greatly reduces previously existing fully formed Abeta1-42 fibrils, producing instead amorphous materials without fibrils but apparently containing some protofibrils and smaller forms. Coincubation of the Abeta1-42 peptide with DAPH produces either amorphous materials or empty fields. Coincubation of DAPH and Abeta1-42 greatly reduces the beta-sheet content, as measured with ThT fluorescence, and produces a novel fluorescent complex with ThT. When the Abeta1-42 peptide was coincubated with DAPH at very low micromolar concentrations, the neuronal toxicity mentioned above (Ca(2+) influx) was eliminated. Clearly, DAPH is a promising candidate for AD therapy.
4,5-Dianilinophthalimide: a protein-tyrosine kinase inhibitor with selectivity for the epidermal growth factor receptor signal transduction pathway and potent in vivo antitumor activity.[Pubmed:8134396]
Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2334-8.
Deregulated signal transduction via the epidermal growth factor receptor (EGF-R) family of protein-tyrosine kinase growth factor receptors is associated with proliferative diseases. We describe a class of compounds (4,5-dianilinophthalimides) that inhibit the EGF-R protein-tyrosine kinase in vitro with high selectivity. In cells, 4,5-dianilinophthalmide selectively inhibited both ligand-induced EGF-R and p185c-erbB2 autophosphorylation and c-fos mRNA induction. Antitumor activity could be demonstrated in vivo against xenografts of the A431 and SK-OV-3 tumors, which overexpress the EGF-R and p185c-erbB2, respectively. In contrast, a platelet-derived growth factor-driven tumor was not inhibited by 4,5-dianilinophthalimide, which is compatible with its cellular selectivity and hypothesized mechanism of action. No overt cumulative toxicity was observed during treatment even though high efficacy was observed, indicating a good therapeutic window. 4,5-Dianilinophthalimides may offer therapeutic agents for the treatment of hyperproliferative diseases that overexpress EGF-R family protein-tyrosine kinases or their ligands.


