SH-4-54STAT inhibitor, potent CAS# 1456632-40-8 |
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- (R)-Crizotinib
Catalog No.:BCC1284
CAS No.:877399-52-5
- Tivantinib (ARQ 197)
Catalog No.:BCC3688
CAS No.:905854-02-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1456632-40-8 | SDF | Download SDF |
| PubChem ID | 72188643 | Appearance | Powder |
| Formula | C29H27F5N2O5S | M.Wt | 610.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (163.78 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
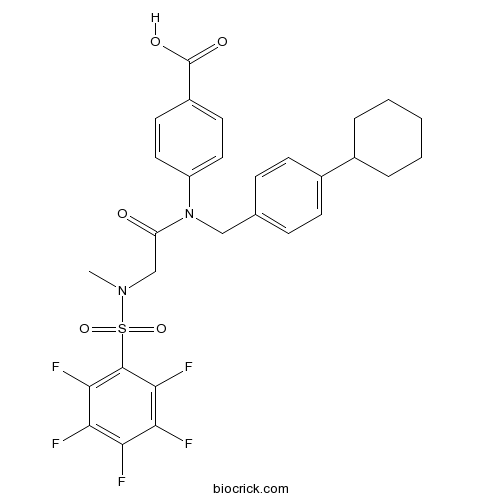
| Chemical Name | 4-[(4-cyclohexylphenyl)methyl-[2-[methyl-(2,3,4,5,6-pentafluorophenyl)sulfonylamino]acetyl]amino]benzoic acid | ||
| SMILES | CN(CC(=O)N(CC1=CC=C(C=C1)C2CCCCC2)C3=CC=C(C=C3)C(=O)O)S(=O)(=O)C4=C(C(=C(C(=C4F)F)F)F)F | ||
| Standard InChIKey | VFPYGNNOSJWBHF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H27F5N2O5S/c1-35(42(40,41)28-26(33)24(31)23(30)25(32)27(28)34)16-22(37)36(21-13-11-20(12-14-21)29(38)39)15-17-7-9-19(10-8-17)18-5-3-2-4-6-18/h7-14,18H,2-6,15-16H2,1H3,(H,38,39) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SH-4-54 is a most potent, small molecule, nonphosphorylated STAT3 inhibitor, strongly binds to STAT3 protein (KD = 300 nM), is selective for STAT3 over STAT1.
IC50 value: 300 nM (KD, for STAT3)
Target: STAT3
in vitro: SH-4-54 potently kills glioblastoma brain cancer stem cells (BTSCs) and effectively suppresses STAT3 phosphorylation and its downstream transcriptional targets at low nM concentrations.SH-4-54 shows unprecedented cytotoxicity in human BTSCs, displayed no toxicity in human fetal astrocytes, potently suppressed pSTAT3 with nanomolar IC50s, inhibited STAT3's downstream targets, and showed no discernible off-target effects at therapeutic doses.
in vivo: SH-4-54 exhibits blood-brain barrier permeability, potently controlled glioma tumor growth, and inhibited pSTAT3 in vivo. SH-4-54 demonstrates the power of STAT3 inhibitors for the treatment of BTSCs and validates the therapeutic efficacy of a STAT3 inhibitor for GBM clinical application.SH-4-54 decreases pSTAT3 expression in tumor cells of treated mice. SH-4-54 appears to decrease proliferation and increase apoptosis of treated tumors. References: | |||||

SH-4-54 Dilution Calculator

SH-4-54 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6378 mL | 8.1888 mL | 16.3776 mL | 32.7552 mL | 40.944 mL |
| 5 mM | 0.3276 mL | 1.6378 mL | 3.2755 mL | 6.551 mL | 8.1888 mL |
| 10 mM | 0.1638 mL | 0.8189 mL | 1.6378 mL | 3.2755 mL | 4.0944 mL |
| 50 mM | 0.0328 mL | 0.1638 mL | 0.3276 mL | 0.6551 mL | 0.8189 mL |
| 100 mM | 0.0164 mL | 0.0819 mL | 0.1638 mL | 0.3276 mL | 0.4094 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SH-4-54 is a potent inhibitor of STAT3 with KD values of 300 and 464 nM for STAT3 and STAT5, respectively [1].
STAT3 is a transcription factor that belongs to the STAT transcription factor family. STAT3 signaling plays an important role in the survival and proliferation of brain tumor stem cells (BTSCs) and glioblastoma (GBM) [1].
SH-4-54 is a potent STAT inhibitor. SH-4-54 inhibited interactions between STAT3 and phosphopeptide with Ki value of 10-30 μM. SH-4-54 exhibited selectivity for STAT3 against STAT1 by >10-fold. SH-4-54 inhibited pSTAT3 (Y705) in a concentration-dependent way with no effect on pSTAT3 (S727), suggesting that SH-4-54 bound to the STAT3 SH2 domain. Also, SH-4-54 reduced pSTAT3 levels and inhibited the downstream targets Cyclin D1 and Bcl-xL, which were involved in cell growth and survival [1].
In mice, intraperitoneal injection of SH-4-54 (10 mg/kg) caused a concentration of 700 nM after 30 min in the brain. In NOD-SCID mice xenografted with 105 BT73 glioma cells, SH-4-54 (10 mg/kg) reduced tumor cells and pSTAT3 expression. Also, SH-4-54 inhibited proliferation and induced apoptosis [1].
Reference:
[1]. Haftchenary S, Luchman HA, Jouk AO, et al. Potent Targeting of the STAT3 Protein in Brain Cancer Stem Cells: A Promising Route for Treating Glioblastoma. ACS Med Chem Lett, 2013, 4(11): 1102-1107.
- 2'-O-Benzoylpaeoniflorin
Catalog No.:BCN7803
CAS No.:1456598-64-3
- NNC 711
Catalog No.:BCC7176
CAS No.:145645-62-1
- Cyclocommunol
Catalog No.:BCN3375
CAS No.:145643-96-5
- Sophocarpine
Catalog No.:BCN5971
CAS No.:145572-44-7
- Eucamalol
Catalog No.:BCN1648
CAS No.:145544-91-8
- Mitiglinide Calcium
Catalog No.:BCC5000
CAS No.:145525-41-3
- 1,7-Dimethoxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7539
CAS No.:145523-71-3
- (2R,3S)-Boc-3-Phenylisoserine
Catalog No.:BCN8362
CAS No.:145514-62-1
- MRS 2179 tetrasodium salt
Catalog No.:BCC5685
CAS No.:1454889-37-2
- PF-06463922
Catalog No.:BCC5568
CAS No.:1454846-35-5
- 4-Benzoylpyridine
Catalog No.:BCC8697
CAS No.:14548-46-0
- LY3009120
Catalog No.:BCC3985
CAS No.:1454682-72-4
- HG-9-91-01
Catalog No.:BCC4071
CAS No.:1456858-58-4
- 3-Allylrhodanine
Catalog No.:BCC8604
CAS No.:1457-47-2
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
- 4,6-Dichloro-5-nitro-2-propylthiopyrimidine
Catalog No.:BCC8667
CAS No.:145783-14-8
- 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine
Catalog No.:BCC8666
CAS No.:145783-15-9
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- Margatoxin
Catalog No.:BCC7709
CAS No.:145808-47-5
- Tiagabine hydrochloride
Catalog No.:BCC5217
CAS No.:145821-59-6
- D-myo-Inositol-1,3,4,5-tetrakisphosphate, octapotassium salt
Catalog No.:BCC7058
CAS No.:145843-69-2
- Brachynoside
Catalog No.:BCN3749
CAS No.:145898-87-9
- CGP 52411
Catalog No.:BCC7667
CAS No.:145915-58-8
- CGP 53353
Catalog No.:BCC7363
CAS No.:145915-60-2
Luteolin-Mediated Inhibition of Hepatic Stellate Cell Activation via Suppression of the STAT3 Pathway.[Pubmed:29795016]
Int J Mol Sci. 2018 May 24;19(6). pii: ijms19061567.
Hepatic stellate cell (HSC) activation is responsible for hepatic fibrogenesis and is associated with an overexpression of transcription 3 (STAT3). Luteolin, a common dietary flavonoid with potent anti-inflammatory properties, has previously demonstrated antifibrogenic properties in HSCs but the mechanism has not been fully elucidated. Activated human and rat hepatic stellate cell lines LX-2 and HSC-T6 were used to study the effects of luteolin on HSCs. Cellular proteins were determined by western blot and immunofluorescence. Cell proliferation was assessed with Alamar Blue assay. Luteolin significantly decreased LX-2 and HSC-T6 cell viability in a time-and-dose-dependent manner, as well as decreased HSC end-products alpha-smooth muscle actin (alpha-SMA), collagen I, and fibronectin. Luteolin decreased levels of total and phosphorylated STAT3, suppressed STAT3 nuclear translocation and transcriptional activity, and attenuated expression of STAT3-regulated proteins c-myc and cyclin D1. STAT3 specific inhibitors stattic and SH-4-54 demonstrated similar effects on HSC viability and alpha-SMA production. In LX-2 and HSC-T6 cells, luteolin demonstrates a potent ability to inhibit hepatic fibrogenesis via suppression of the STAT3 pathway. These results further elucidate the mechanism of luteolin as well as the effect of the STAT3 pathway on HSC activation.
Presence of stromal cells in a bioengineered tumor microenvironment alters glioblastoma migration and response to STAT3 inhibition.[Pubmed:29566069]
PLoS One. 2018 Mar 22;13(3):e0194183.
Despite the increasingly recognized importance of the tumor microenvironment (TME) as a regulator of tumor progression, only few in vitro models have been developed to systematically study the effects of TME on tumor behavior in a controlled manner. Here we developed a three-dimensional (3D) in vitro model that recapitulates the physical and compositional characteristics of Glioblastoma (GBM) extracellular matrix (ECM) and incorporates brain stromal cells such as astrocytes and endothelial cell precursors. The model was used to evaluate the effect of TME components on migration and survival of various patient-derived GBM cell lines (GBM10, GBM43 and GBAM1) in the context of STAT3 inhibition. Migration analysis of GBM within the 3D in vitro model demonstrated that the presence of astrocytes significantly increases the migration of GBM, while presence of endothelial precursors has varied effects on the migration of different GBM cell lines. Given the role of the tumor microenvironment as a regulator of STAT3 activity, we tested the effect of the STAT3 inhibitor SH-4-54 on GBM migration and survival. SH-4-54 inhibited STAT3 activity and reduced 3D migration and survival of GBM43 but had no effect on GBM10. SH-4-54 treatment drastically reduced the viability of the stem-like line GBAM1 in liquid culture, but its effect lessened in presence of a 3D ECM and stromal cells. Our results highlight the interplay between the ECM and stromal cells in the microenvironment with the cancer cells and indicate that the impact of these relationships may differ for GBM cells of varying genetic and clinical histories.
Persistent STAT5-mediated ROS production and involvement of aberrant p53 apoptotic signaling in the resistance of chronic myeloid leukemia to imatinib.[Pubmed:29115375]
Int J Mol Med. 2018 Jan;41(1):455-463.
The persistent activation of signal transducer and activator of transcription 5 (STAT5) may principally be attributed to breakpoint cluster region (BCR)-Abelson murine leukemia viral oncogene homolog 1 (ABL1), and have multi-faceted effects in the development of chronic myeloid leukemia (CML). The p53 protein network regulates important mechanisms in DNA damage repair, cell cycle regulation/checkpoints, and cell senescence and apoptosis, as demonstrated by its ability to positively regulate the expression of various pro-apoptotic genes, including B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein (Bax). In the present study, it was observed that the mRNA levels of STAT5A and STAT5B were upregulated in patients with imatinib-resistant CML and in the imatinib-resistant K562/G CML cell line. In addition, increased expression of STAT5 was observed in the BCR-ABL1 mutation group, compared with that in the non-BCR-ABL1 mutation group, regardless of patient imatinib resistance state. Elevated levels of reactive oxygen species (ROS) and DNA double-strand breaks were identified in K562/G cells using flow cytometric and phosphorylated H2AX (gamma-H2AX) foci immunofluorescence assays, respectively, compared with the imatinib-sensitive K562 cells. The levels of intracellular ROS and gamma-H2AX were decreased by the ROS scavenger (N-acetylcysteine), and ROS levels were also markedly reduced by STAT5 inhibitor (SH-4-54). In addition, imatinib significantly inhibited the proliferation of K562 and K562/G cells, with half maximal inhibitory concentration values of 0.17+/-0.07 and 14.78+/-0.43 microM, respectively, and the levels of apoptosis were significantly different between K562 and K562/G cells following treatment with imatinib. The mRNA and protein levels of STAT5 and mouse double minute 2 homolog (MDM2) were upregulated, whereas those of Bax were downregulated in K562/G cells, as determined using western blot analysis. Additionally, although the two cell lines exhibited relatively low protein expression levels of p53, lower levels of p53 and TPp53BP1 transcripts were detected in the K562/G cells. Taken together, these findings suggest that the resistance of CML to the tyrosine kinase inhibitor, imatinib, may be associated with persistent STAT5-mediated ROS production, and the abnormality of the p53 pathway.
Chronic Inhibition of STAT3/STAT5 in Treatment-Resistant Human Breast Cancer Cell Subtypes: Convergence on the ROS/SUMO Pathway and Its Effects on xCT Expression and System xc- Activity.[Pubmed:27513743]
PLoS One. 2016 Aug 11;11(8):e0161202.
Pharmacologically targeting activated STAT3 and/or STAT5 has been an active area of cancer research. The cystine/glutamate antiporter, system xc-, contributes to redox balance and export of intracellularly produced glutamate in response to up-regulated glutaminolysis in cancer cells. We have previously shown that blocking STAT3/5 using the small molecule inhibitor, SH-4-54, which targets the SH2 domains of both proteins, increases xCT expression, thereby increasing system xc- activity in human breast cancer cells. The current investigation demonstrates that chronic SH-4-54 administration, followed by clonal selection of treatment-resistant MDA-MB-231 and T47D breast cancer cells, elicits distinct subtype-dependent effects. xCT mRNA and protein levels, glutamate release, and cystine uptake are decreased relative to untreated passage-matched controls in triple-negative MDA-MB-231 cells, with the inverse occurring in estrogen-responsive T47D cells. This "ying-yang" effect is linked with a shifted balance between the phosphorylation status of STAT3 and STAT5, intracellular ROS levels, and STAT5 SUMOylation/de-SUMOylation. STAT5 emerged as a definitive negative regulator of xCT at the transcriptional level, while STAT3 activation is coupled with increased system xc- activity. We propose that careful classification of a patient's breast cancer subtype is central to effectively targeting STAT3/5 as a therapeutic means of treating breast cancer, particularly given that xCT is emerging as an important biomarker of aggressive cancers.
Applying Small Molecule Signal Transducer and Activator of Transcription-3 (STAT3) Protein Inhibitors as Pancreatic Cancer Therapeutics.[Pubmed:26873728]
Mol Cancer Ther. 2016 May;15(5):794-805.
Constitutively activated STAT3 protein has been found to be a key regulator of pancreatic cancer and a target for molecular therapeutic intervention. In this study, PG-S3-001, a small molecule derived from the SH-4-54 class of STAT3 inhibitors, was found to inhibit patient-derived pancreatic cancer cell proliferation in vitro and in vivo in the low micromolar range. PG-S3-001 binds the STAT3 protein potently, Kd = 324 nmol/L by surface plasmon resonance, and showed no effect in a kinome screen (>100 cancer-relevant kinases). In vitro studies demonstrated potent cell killing as well as inhibition of STAT3 activation in pancreatic cancer cells. To better model the tumor and its microenvironment, we utilized three-dimensional (3D) cultures of patient-derived pancreatic cancer cells in the absence and presence of cancer-associated fibroblasts (CAF). In this coculture model, inhibition of tumor growth is maintained following STAT3 inhibition in the presence of CAFs. Confocal microscopy was used to verify tumor cell death following treatment of 3D cocultures with PG-S3-001. The 3D model was predictive of in vivo efficacy as significant tumor growth inhibition was observed upon administration of PG-S3-001. These studies showed that the inhibition of STAT3 was able to impact the survival of tumor cells in a relevant 3D model, as well as in a xenograft model using patient-derived cells. Mol Cancer Ther; 15(5); 794-805. (c)2016 AACR.
Potent Targeting of the STAT3 Protein in Brain Cancer Stem Cells: A Promising Route for Treating Glioblastoma.[Pubmed:24900612]
ACS Med Chem Lett. 2013 Sep 8;4(11):1102-7.
The STAT3 gene is abnormally active in glioblastoma (GBM) and is a critically important mediator of tumor growth and therapeutic resistance in GBM. Thus, for poorly treated brain cancers such as gliomas, astrocytomas, and glioblastomas, which harbor constitutively activated STAT3, a STAT3-targeting therapeutic will be of significant importance. Herein, we report a most potent, small molecule, nonphosphorylated STAT3 inhibitor, 31 (SH-4-54) that strongly binds to STAT3 protein (K D = 300 nM). Inhibitor 31 potently kills glioblastoma brain cancer stem cells (BTSCs) and effectively suppresses STAT3 phosphorylation and its downstream transcriptional targets at low nM concentrations. Moreover, in vivo, 31 exhibited blood-brain barrier permeability, potently controlled glioma tumor growth, and inhibited pSTAT3 in vivo. This work, for the first time, demonstrates the power of STAT3 inhibitors for the treatment of BTSCs and validates the therapeutic efficacy of a STAT3 inhibitor for GBM clinical application.


