EucamalolCAS# 145544-91-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145544-91-8 | SDF | Download SDF |
| PubChem ID | 91884957 | Appearance | Oil |
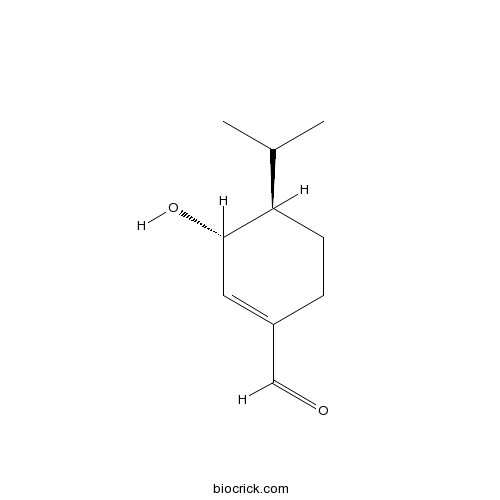
| Formula | C10H16O2 | M.Wt | 168.2 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R,4R)-3-hydroxy-4-propan-2-ylcyclohexene-1-carbaldehyde | ||
| SMILES | CC(C)C1CCC(=CC1O)C=O | ||
| Standard InChIKey | ZPACRXLIAKZISA-ZJUUUORDSA-N | ||
| Standard InChI | InChI=1S/C10H16O2/c1-7(2)9-4-3-8(6-11)5-10(9)12/h5-7,9-10,12H,3-4H2,1-2H3/t9-,10+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Eucamolol exhibits significant repellent activity against Aedes albopictus, and inhibits its feeding as well as DEET, is effective repellent (75%) up to 3 h after exposure to mosquito. |

Eucamalol Dilution Calculator

Eucamalol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9453 mL | 29.7265 mL | 59.453 mL | 118.9061 mL | 148.6326 mL |
| 5 mM | 1.1891 mL | 5.9453 mL | 11.8906 mL | 23.7812 mL | 29.7265 mL |
| 10 mM | 0.5945 mL | 2.9727 mL | 5.9453 mL | 11.8906 mL | 14.8633 mL |
| 50 mM | 0.1189 mL | 0.5945 mL | 1.1891 mL | 2.3781 mL | 2.9727 mL |
| 100 mM | 0.0595 mL | 0.2973 mL | 0.5945 mL | 1.1891 mL | 1.4863 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Mitiglinide Calcium
Catalog No.:BCC5000
CAS No.:145525-41-3
- 1,7-Dimethoxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7539
CAS No.:145523-71-3
- (2R,3S)-Boc-3-Phenylisoserine
Catalog No.:BCN8362
CAS No.:145514-62-1
- MRS 2179 tetrasodium salt
Catalog No.:BCC5685
CAS No.:1454889-37-2
- PF-06463922
Catalog No.:BCC5568
CAS No.:1454846-35-5
- 4-Benzoylpyridine
Catalog No.:BCC8697
CAS No.:14548-46-0
- LY3009120
Catalog No.:BCC3985
CAS No.:1454682-72-4
- Furano(2'',3'',7,6)-4'-hydroxyflavanone
Catalog No.:BCN6405
CAS No.:1454619-70-5
- PU-WS13
Catalog No.:BCC6425
CAS No.:1454619-14-7
- Isolintetralin
Catalog No.:BCN3052
CAS No.:145459-30-9
- Heterophyllin B
Catalog No.:BCN2768
CAS No.:145459-19-4
- L-692,585
Catalog No.:BCC7305
CAS No.:145455-35-2
- Sophocarpine
Catalog No.:BCN5971
CAS No.:145572-44-7
- Cyclocommunol
Catalog No.:BCN3375
CAS No.:145643-96-5
- NNC 711
Catalog No.:BCC7176
CAS No.:145645-62-1
- 2'-O-Benzoylpaeoniflorin
Catalog No.:BCN7803
CAS No.:1456598-64-3
- SH-4-54
Catalog No.:BCC5483
CAS No.:1456632-40-8
- HG-9-91-01
Catalog No.:BCC4071
CAS No.:1456858-58-4
- 3-Allylrhodanine
Catalog No.:BCC8604
CAS No.:1457-47-2
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
- 4,6-Dichloro-5-nitro-2-propylthiopyrimidine
Catalog No.:BCC8667
CAS No.:145783-14-8
- 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine
Catalog No.:BCC8666
CAS No.:145783-15-9
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- Margatoxin
Catalog No.:BCC7709
CAS No.:145808-47-5
Absolute configuration of a new mosquito repellent, (+)-eucamalol and the repellent activity of its epimer.[Pubmed:7613002]
Biosci Biotechnol Biochem. 1995 Jun;59(6):1139-41.
(+)-Eucamalol (1) and (-)-1-epi-Eucamalol (2) were synthesized from (S)-(-)-perillaldehyde to determine the absolute configuration of 1, the structure of natural (+)-Eucamalol being determined to be (1R,6R)-(+)-3-formyl-6-isopropyl-2-cyclohexen-1-ol. (+)-Eucamolol (1) and its 1-epimer (2) exhibited significant repellent activity against Aedes albopictus, and inhibited its feeding as well as DEET.
Polyoxygenated eudesmanes and trans-chrysanthemanes from the aerial parts of Santolina insularis.[Pubmed:14738382]
J Nat Prod. 2004 Jan;67(1):37-41.
The eudesmane sesquiterpenoids 1-3 and the trans-chrysanthemyl monoterpenoid 4 have been isolated from the aerial parts of Santolina insularis, a bush endemic to Sardinia. The absolute stereostructures of these novel compounds and of two known but incompletely characterized chrysanthemanes (5, 6) were established by spectroscopic techniques and by application of the modified Mosher method. The presence of the p-menthane aldehyde Eucamalol (7) gives credit to the widespread use of S. insularis to fend off mosquitoes.


