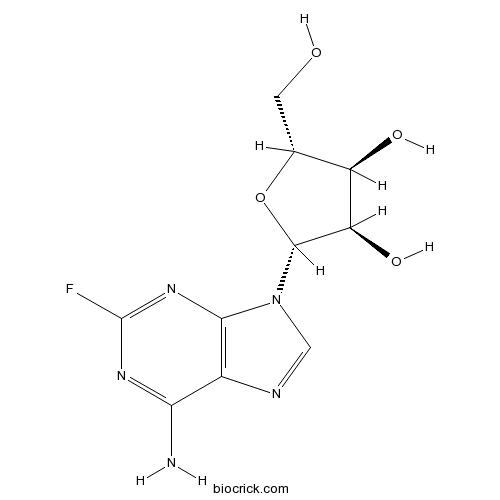
2-FluoroadenosineCAS# 146-78-1 |
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 146-78-1 | SDF | Download SDF |
| PubChem ID | 8975 | Appearance | Powder |
| Formula | C10H12FN5O4 | M.Wt | 285 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4S,5R)-2-(6-amino-2-fluoropurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | C1=NC2=C(N1C3C(C(C(O3)CO)O)O)N=C(N=C2N)F | ||
| Standard InChIKey | HBUBKKRHXORPQB-UUOKFMHZSA-N | ||
| Standard InChI | InChI=1S/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2-Fluoroadenosine Dilution Calculator

2-Fluoroadenosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5088 mL | 17.5439 mL | 35.0877 mL | 70.1754 mL | 87.7193 mL |
| 5 mM | 0.7018 mL | 3.5088 mL | 7.0175 mL | 14.0351 mL | 17.5439 mL |
| 10 mM | 0.3509 mL | 1.7544 mL | 3.5088 mL | 7.0175 mL | 8.7719 mL |
| 50 mM | 0.0702 mL | 0.3509 mL | 0.7018 mL | 1.4035 mL | 1.7544 mL |
| 100 mM | 0.0351 mL | 0.1754 mL | 0.3509 mL | 0.7018 mL | 0.8772 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-Chloroadenosine
Catalog No.:BCC7575
CAS No.:146-77-0
- Yohimbine
Catalog No.:BCN2293
CAS No.:146-48-5
- Cycloart-23-ene-3,25-diol
Catalog No.:BCN2640
CAS No.:14599-48-5
- Jasminoid A
Catalog No.:BCN7605
CAS No.:1459784-57-6
- Laccaic acid E
Catalog No.:BCN1807
CAS No.:14597-16-1
- N,N,N-Trimethyl-2-aminoethylphosphonate
Catalog No.:BCN1560
CAS No.:14596-57-7
- 2-Dimethylaminoethylphosphonic acid
Catalog No.:BCN1764
CAS No.:14596-56-6
- 2-(Methylamino)ethylphosphonic acid
Catalog No.:BCN1763
CAS No.:14596-55-5
- 3-Amino-3-phenyl-1-propanol
Catalog No.:BCC8608
CAS No.:14593-04-5
- 7,8,9,9-Tetradehydroisolariciresinol
Catalog No.:BCN1649
CAS No.:145918-59-8
- CGP 53353
Catalog No.:BCC7363
CAS No.:145915-60-2
- CGP 52411
Catalog No.:BCC7667
CAS No.:145915-58-8
- Tropine nonanoate
Catalog No.:BCN1925
CAS No.:146018-90-8
- SC 51322
Catalog No.:BCC5941
CAS No.:146032-79-3
- SC 51089
Catalog No.:BCC7773
CAS No.:146033-02-5
- Tauroursodeoxycholic acid
Catalog No.:BCN6953
CAS No.:14605-22-2
- MSDC-0160
Catalog No.:BCC5343
CAS No.:146062-49-9
- Dihydromarein
Catalog No.:BCN8406
CAS No.:
- Pulchinenoside E1
Catalog No.:BCN8185
CAS No.:146100-02-9
- Z-Arg(Z)2-OH
Catalog No.:BCC3574
CAS No.:14611-34-8
- R-(-)-Deprenyl hydrochloride
Catalog No.:BCC5196
CAS No.:14611-52-0
- Chlorajapolide F
Catalog No.:BCN6425
CAS No.:1461760-59-7
- N-Methyllidocaine iodide
Catalog No.:BCC6905
CAS No.:1462-71-1
- SR 48692
Catalog No.:BCC7763
CAS No.:146362-70-1
A Cascade of Thermophilic Enzymes As an Approach to the Synthesis of Modified Nucleotides.[Pubmed:28050269]
Acta Naturae. 2016 Oct-Dec;8(4):82-90.
We propose a new approach for the synthesis of biologically important nucleotides which includes a multi-enzymatic cascade conversion of D-pentoses into purine nucleotides. The approach exploits nucleic acid exchange enzymes from thermophilic microorganisms: ribokinase, phosphoribosylpyrophosphate synthetase, and adenine phosphoribosyltransferase. We cloned the ribokinase gene from Thermus sp. 2.9, as well as two different genes of phosphoribosylpyrophosphate synthetase (PRPP-synthetase) and the adenine phosphoribosyltransferase (APR-transferase) gene from Thermus thermophilus HB27 into the expression vectors, generated high-yield E. coli producer strains, developed methods for the purification of the enzymes, and investigated enzyme substrate specificity. The enzymes were used for the conversion of D-pentoses into 5-phosphates that were further converted into 5-phospho-alpha-D-pentofuranose 1-pyrophosphates by means of ribokinase and PRPP-synthetases. Target nucleotides were obtained through the condensation of the pyrophosphates with adenine and its derivatives in a reaction catalyzed by APR-transferase. 2-Chloro- and 2-Fluoroadenosine monophosphates were synthesized from D-ribose and appropriate heterobases in one pot using a system of thermophilic enzymes in the presence of ATP, ribokinase, PRPP-synthetase, and APR-transferase.
(1)(9)F-Site-Specific-Labeled Nucleotides for Nucleic Acid Structural Analysis by NMR.[Pubmed:26791976]
Methods Enzymol. 2016;566:59-87.
Naturally occurring RNA lacks fluorine-19 ((19)F), thus, their specifically fluorinated counterparts are particularly well suited to noninvasively monitoring the dynamic conformational properties and ligand-binding interactions of the RNA. For nuclear magnetic resonance (NMR) spectroscopy, (19)F-NMR of fluorine-substituted RNA provides an attractive, site-specific probe for structure determination in solution. Advantages of (19)F include high NMR sensitivity (83% of (1)H), high natural abundance (100%), and the extreme sensitivity of (19)F to the chemical environment leading to a large range of chemical shifts. The preparation of base-substituted 2-fluoropurine and 5-fluoropyrimidine 5'-triphosphates (2F-ATP/5F-CTP/5F-UTP) can be carried out using efficient enzymatic synthesis methods. Both pyrimidine analogs, 5-fluorouridine and 5-fluorocytidine, as well as, 2-Fluoroadenosine are readily incorporated into RNA transcribed in vitro using T7 RNA polymerase.
(19)F-labeling of the adenine H2-site to study large RNAs by NMR spectroscopy.[Pubmed:26704707]
J Biomol NMR. 2016 Jan;64(1):63-74.
In comparison to proteins and protein complexes, the size of RNA amenable to NMR studies is limited despite the development of new isotopic labeling strategies including deuteration and ligation of differentially labeled RNAs. Due to the restricted chemical shift dispersion in only four different nucleotides spectral resolution remains limited in larger RNAs. Labeling RNAs with the NMR-active nucleus (19)F has previously been introduced for small RNAs up to 40 nucleotides (nt). In the presented work, we study the natural occurring RNA aptamer domain of the guanine-sensing riboswitch comprising 73 nucleotides from Bacillus subtilis. The work includes protocols for improved in vitro transcription of 2-Fluoroadenosine-5'-triphosphat (2F-ATP) using the mutant P266L of the T7 RNA polymerase. Our NMR analysis shows that the secondary and tertiary structure of the riboswitch is fully maintained and that the specific binding of the cognate ligand hypoxanthine is not impaired by the introduction of the (19)F isotope. The thermal stability of the (19)F-labeled riboswitch is not altered compared to the unmodified sequence, but local base pair stabilities, as measured by hydrogen exchange experiments, are modulated. The characteristic change in the chemical shift of the imino resonances detected in a (1)H,(15)N-HSQC allow the identification of Watson-Crick base paired uridine signals and the (19)F resonances can be used as reporters for tertiary and secondary structure transitions, confirming the potential of (19)F-labeling even for sizeable RNAs in the range of 70 nucleotides.
Isolation and substrate specificity of an adenine nucleoside phosphorylase from adult Schistosoma mansoni.[Pubmed:24794680]
Mol Biochem Parasitol. 2014 Mar-Apr;194(1-2):44-7.
An adenine nucleoside phosphorylase (ANP, EC none) activity was identified and partially purified from extracts of Schistosoma mansoni by chromatofocussing column chromatography and molecular sieving. The enzyme is distinct from purine nucleoside phosphorylase (PNP, EC 2.4.2.1). ANP is specific for adenine nucleosides which includes adenosine analogs modified in the aglycone, pentose or both moieties. (e.g. 2'-deoxyadenosine, 5'-deoxy-5'-methylthioadenosine, 5'-deoxy-5'-iodo-2-Fluoroadenosine, etc.) The enzyme is also distinct from the mammalian 5'-deoxy-5'-methylthioadenosine phosphorylase (MTAP, EC 2.4.2.28) in that it is able of the phosphorolysis of 2'-deoxyadenosine while mammalian MTAP cannot. Because of ANP unique substrate specificity, the enzyme could play a role as a target for chemotherapy of these parasites. Cytotoxic analogs may be designed as subversive substrates that are selectively activated only by the schistosomal ANP.
Adenosine kinase from Schistosoma mansoni: structural basis for the differential incorporation of nucleoside analogues.[Pubmed:23275171]
Acta Crystallogr D Biol Crystallogr. 2013 Jan;69(Pt 1):126-36.
In adult schistosomes, the enzyme adenosine kinase (AK) is responsible for the incorporation of some adenosine analogues, such as 2-Fluoroadenosine and tubercidin, into the nucleotide pool, but not others. In the present study, the structures of four complexes of Schistosoma mansoni AK bound to adenosine and adenosine analogues are reported which shed light on this observation. Two differences in the adenosine-binding site in comparison with the human counterpart (I38Q and T36A) are responsible for their differential specificities towards adenosine analogues, in which the Schistosoma enzyme does not tolerate bulky substituents at the N7 base position. This aids in explaining experimental data which were reported in the literature more than two decades ago. Furthermore, there appears to be considerable plasticity within the substrate-binding sites that affects the side-chain conformation of Ile38 and causes a previously unobserved flexibility within the loop comprising residues 286-299. These results reveal that the latter can be sterically occluded in the absence of ATP. Overall, these results contribute to the body of knowledge concerning the enzymes of the purine salvage pathway in this important human parasite.
Development of modified nucleosides that have supremely high anti-HIV activity and low toxicity and prevent the emergence of resistant HIV mutants.[Pubmed:21422739]
Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(3):53-65.
An idea to use 4'-C-substituted-2'-deoxynucleoside derivatives was proposed based on a working hypothesis to solve the problems of existing acquired immune deficiency syndrome chemotherapy (highly active antiretroviral therapy). Subsequent studies have successfully proved the validity of the idea and resulted in the development of 2'-deoxy-4'-C-ethynyl-2-Fluoroadenosine and 2'-deoxy-4'-C-ethynyl-2-chloroadenosine, nucleoside reverse transcriptase inhibitors, which have supremely high activity against all human immunodeficiency viruses including multidrug-resistant HIV and low toxicity.
In vitro assessment of anticryptosporidial efficacy and cytotoxicity of adenosine analogues using a SYBR Green real-time PCR method.[Pubmed:21393228]
J Antimicrob Chemother. 2011 Mar;66(3):560-3.
OBJECTIVES: The aims of this study were to provide a cost-effective and valuable method for evaluating drug efficacy against Cryptosporidium parvum using a quantitative SYBR Green real-time PCR (qPCR) and to assess the efficacy of adenosine analogues as drug templates. METHODS: C. parvum HNJ-1 strain growing in human ileocaecal adenocarcinoma cells was employed as an in vitro culture system. To normalize the DNA extraction efficiency, a specific plasmid was added to each sample before DNA purification; the genomic DNA of infected cells was quantified by qPCR using specific primers to confirm drug efficacy and cytotoxicity. To determine the mechanism of action, enzymatic inhibition analyses were conducted using C. parvum S-adenosyl-l-homocysteine hydrolase (CpSAHH) recombinant protein. RESULTS: The dose-dependent growth inhibition of C. parvum was confirmed; 50% effective concentrations of neplanocin A (NPA) and 2-Fluoroadenosine (2FA) were 139 muM and 0.842 muM, respectively. Cytotoxicity evaluation showed that the 50% growth inhibition concentration of 2FA was 1.18 muM; NPA did not exhibit any cytotoxicity up to 200 muM. The screening system revealed the specific but marginal efficacy of NPA and showed 2FA to be cytotoxic. Recombinant CpSAHH inhibition analyses showed that NPA competitively inhibited CpSAHH activity (K(i )= 0.395 muM), whereas 2FA did not. CONCLUSIONS: This novel qPCR system confirmed not only drug efficacy against C. parvum but also cytotoxicity to host cells. Moreover, since the SYBR Green method is cost effective, it could therefore be used in a wide variety of clinical and research-oriented applications of Cryptosporidium analysis.
[The preparative method for 2-fluoroadenosine synthesis].[Pubmed:19537172]
Bioorg Khim. 2009 Mar-Apr;35(2):210-4.
The preparative method for the synthesis of 2-Fluoroadenosine starting from commercially available guanosine was developed. It included the intermediate formation of 2-amino-6-azido-9-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)purine, which was isolated exclusively in the tetrazolo[5,1-i]-form {5-amino-7-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)-7H-tetrazolo[5,1-i]purine}. The latter compound was converted by the Schiemann reaction to 6-azido-2-fluoro-9-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)purine, which was isolated at an 80% yield after careful optimization of the process. The IR and 1H NMR spectroscopy data indicated the 6-azido-2-fluoropurine structure of the aglycone. The catalytic reduction of the azido group in 6-azido-2-fluoro-9-(2,3,5-tri-O-acetyl-beta-D-ribofuranosyl)purine to the amino moiety and the subsequent deacetylation by the routine procedure resulted in 2-Fluoroadenosine at a total yield of 74%.
Crystal structures of Mycobacterium tuberculosis S-adenosyl-L-homocysteine hydrolase in ternary complex with substrate and inhibitors.[Pubmed:18815415]
Protein Sci. 2008 Dec;17(12):2134-44.
S-adenosylhomocysteine hydrolase (SAHH) is a ubiquitous enzyme that plays a central role in methylation-based processes by maintaining the intracellular balance between S-adenosylhomocysteine (SAH) and S-adenosylmethionine. We report the first prokaryotic crystal structure of SAHH, from Mycobacterium tuberculosis (Mtb), in complex with adenosine (ADO) and nicotinamide adenine dinucleotide. Structures of complexes with three inhibitors are also reported: 3'-keto aristeromycin (ARI), 2-Fluoroadenosine, and 3-deazaadenosine. The ARI complex is the first reported structure of SAHH complexed with this inhibitor, and confirms the oxidation of the 3' hydroxyl to a planar keto group, consistent with its prediction as a mechanism-based inhibitor. We demonstrate the in vivo enzyme inhibition activity of the three inhibitors and also show that 2-fluoradenosine has bactericidal activity. While most of the residues lining the ADO-binding pocket are identical between Mtb and human SAHH, less is known about the binding mode of the homocysteine (HCY) appendage of the full substrate. We report the 2.0 A resolution structure of the complex of SAHH cocrystallized with SAH. The most striking change in the structure is that binding of HCY forces a rotation of His363 around the backbone to flip out of contact with the 5' hydroxyl of the ADO and opens access to a nearby channel that leads to the surface. This complex suggests that His363 acts as a switch that opens up to permit binding of substrate, then closes down after release of the cleaved HCY. Differences in the entrance to this access channel between human and Mtb SAHH are identified.
2'-deoxy-4'-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants.[Pubmed:18487070]
Int J Biochem Cell Biol. 2008;40(11):2410-20.
One of the formidable challenges in therapy of infections by human immunodeficiency virus (HIV) is the emergence of drug-resistant variants that attenuate the efficacy of highly active antiretroviral therapy (HAART). We have recently introduced 4'-ethynyl-nucleoside analogs as nucleoside reverse transcriptase inhibitors (NRTIs) that could be developed as therapeutics for treatment of HIV infections. In this study, we present 2'-deoxy-4'-C-ethynyl-2-Fluoroadenosine (EFdA), a second generation 4'-ethynyl inhibitor that exerted highly potent activity against wild-type HIV-1 (EC50 approximately 0.07 nM). EFdA retains potency toward many HIV-1 resistant strains, including the multi-drug resistant clone HIV-1A62V/V75I/F77L/F116Y/Q151M. The selectivity index of EFdA (cytotoxicity/inhibitory activity) is more favorable than all approved NRTIs used in HIV therapy. Furthermore, EFdA efficiently inhibited clinical isolates from patients heavily treated with multiple anti-HIV-1 drugs. EFdA appears to be primarily phosphorylated by the cellular 2'-deoxycytidine kinase (dCK) because: (a) the antiviral activity of EFdA was reduced by the addition of dC, which competes nucleosides phosphorylated by the dCK pathway, (b) the antiviral activity of EFdA was significantly reduced in dCK-deficient HT-1080/Ara-Cr cells, but restored after dCK transduction. Further, unlike other dA analogs, EFdA is completely resistant to degradation by adenosine deaminase. Moderate decrease in susceptibility to EFdA is conferred by a combination of three RT mutations (I142V, T165R, and M184V) that result in a significant decrease of viral fitness. Molecular modeling analysis suggests that the M184V/I substitutions may reduce anti-HIV activity of EFdA through steric hindrance between its 4'-ethynyl moiety and the V/I184 beta-branched side chains. The present data suggest that EFdA, is a promising candidate for developing as a therapeutic agent for the treatment of individuals harboring multi-drug resistant HIV variants.
2'-deoxy-4'-C-ethynyl-2-fluoroadenosine: a nucleoside reverse transcriptase inhibitor with highly potent activity against wide spectrum of HIV-1 strains, favorable toxic profiles, and stability in plasma.[Pubmed:18066823]
Nucleosides Nucleotides Nucleic Acids. 2007;26(10-12):1543-6.
Working hypotheses to solve the critical problems of the existing highly active anti-retroviral therapy were proposed. The study based on the hypotheses proved the validity of the hypotheses and resulted in the development of 2'-deoxy-4'-C-ethynyl-2-Fluoroadenosine, a nucleoside reverse transcriptase inhibitor, with highly potent activity against all HIV-1, very favorable toxic profiles, and stability in plasma. The nucleoside will prevent or delay the emergence of drug-resistant HIV-1 variants and be an ideal therapeutic agent for both HIV-1 and HBV infections.
High resolution crystal structures of Mycobacterium tuberculosis adenosine kinase: insights into the mechanism and specificity of this novel prokaryotic enzyme.[Pubmed:17597075]
J Biol Chem. 2007 Sep 14;282(37):27334-42.
Adenosine kinase (ADK) catalyzes the phosphorylation of adenosine (Ado) to adenosine monophosphate (AMP). It is part of the purine salvage pathway that has been identified only in eukaryotes, with the single exception of Mycobacterium spp. Whereas it is not clear if Mycobacterium tuberculosis (Mtb) ADK is essential, it has been shown that the enzyme can selectively phosphorylate nucleoside analogs to produce products toxic to the cell. We have determined the crystal structure of Mtb ADK unliganded as well as ligand (Ado) bound at 1.5- and 1.9-A resolution, respectively. The structure of the binary complexes with the inhibitor 2-Fluoroadenosine (F-Ado) bound and with the adenosine 5'-(beta,gamma-methylene)triphosphate (AMP-PCP) (non-hydrolyzable ATP analog) bound were also solved at 1.9-A resolution. These four structures indicate that Mtb ADK is a dimer formed by an extended beta sheet. The active site of the unliganded ADK is in an open conformation, and upon Ado binding a lid domain of the protein undergoes a large conformation change to close the active site. In the closed conformation, the lid forms direct interactions with the substrate and residues of the active site. Interestingly, AMP-PCP binding alone was not sufficient to produce the closed state of the enzyme. The binding mode of F-Ado was characterized to illustrate the role of additional non-bonding interactions in Mtb ADK compared with human ADK.


