Laccaic acid ECAS# 14597-16-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14597-16-1 | SDF | Download SDF |
| PubChem ID | 15045881 | Appearance | Yellow powder |
| Formula | C24H17NO11 | M.Wt | 495.40 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
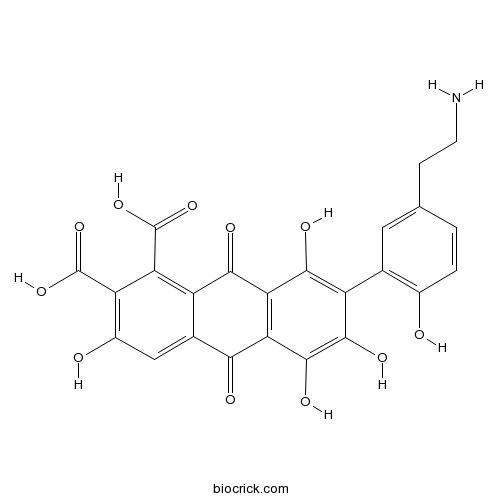
| Chemical Name | 7-[5-(2-aminoethyl)-2-hydroxyphenyl]-3,5,6,8-tetrahydroxy-9,10-dioxoanthracene-1,2-dicarboxylic acid | ||
| SMILES | C1=CC(=C(C=C1CCN)C2=C(C3=C(C(=C2O)O)C(=O)C4=CC(=C(C(=C4C3=O)C(=O)O)C(=O)O)O)O)O | ||
| Standard InChIKey | QPCFHDQETKDGHT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H17NO11/c25-4-3-7-1-2-10(26)8(5-7)13-20(30)17-16(22(32)21(13)31)18(28)9-6-11(27)14(23(33)34)15(24(35)36)12(9)19(17)29/h1-2,5-6,26-27,30-32H,3-4,25H2,(H,33,34)(H,35,36) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Laccaic acids A, B and E as coloring matters in the examined preparations of lac-dye. |

Laccaic acid E Dilution Calculator

Laccaic acid E Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0186 mL | 10.0929 mL | 20.1857 mL | 40.3714 mL | 50.4643 mL |
| 5 mM | 0.4037 mL | 2.0186 mL | 4.0371 mL | 8.0743 mL | 10.0929 mL |
| 10 mM | 0.2019 mL | 1.0093 mL | 2.0186 mL | 4.0371 mL | 5.0464 mL |
| 50 mM | 0.0404 mL | 0.2019 mL | 0.4037 mL | 0.8074 mL | 1.0093 mL |
| 100 mM | 0.0202 mL | 0.1009 mL | 0.2019 mL | 0.4037 mL | 0.5046 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N,N,N-Trimethyl-2-aminoethylphosphonate
Catalog No.:BCN1560
CAS No.:14596-57-7
- 2-Dimethylaminoethylphosphonic acid
Catalog No.:BCN1764
CAS No.:14596-56-6
- 2-(Methylamino)ethylphosphonic acid
Catalog No.:BCN1763
CAS No.:14596-55-5
- 3-Amino-3-phenyl-1-propanol
Catalog No.:BCC8608
CAS No.:14593-04-5
- 7,8,9,9-Tetradehydroisolariciresinol
Catalog No.:BCN1649
CAS No.:145918-59-8
- CGP 53353
Catalog No.:BCC7363
CAS No.:145915-60-2
- CGP 52411
Catalog No.:BCC7667
CAS No.:145915-58-8
- Brachynoside
Catalog No.:BCN3749
CAS No.:145898-87-9
- D-myo-Inositol-1,3,4,5-tetrakisphosphate, octapotassium salt
Catalog No.:BCC7058
CAS No.:145843-69-2
- Tiagabine hydrochloride
Catalog No.:BCC5217
CAS No.:145821-59-6
- Margatoxin
Catalog No.:BCC7709
CAS No.:145808-47-5
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- Jasminoid A
Catalog No.:BCN7605
CAS No.:1459784-57-6
- Cycloart-23-ene-3,25-diol
Catalog No.:BCN2640
CAS No.:14599-48-5
- Yohimbine
Catalog No.:BCN2293
CAS No.:146-48-5
- 2-Chloroadenosine
Catalog No.:BCC7575
CAS No.:146-77-0
- 2-Fluoroadenosine
Catalog No.:BCC8576
CAS No.:146-78-1
- Tropine nonanoate
Catalog No.:BCN1925
CAS No.:146018-90-8
- SC 51322
Catalog No.:BCC5941
CAS No.:146032-79-3
- SC 51089
Catalog No.:BCC7773
CAS No.:146033-02-5
- Tauroursodeoxycholic acid
Catalog No.:BCN6953
CAS No.:14605-22-2
- MSDC-0160
Catalog No.:BCC5343
CAS No.:146062-49-9
- Dihydromarein
Catalog No.:BCN8406
CAS No.:
- Pulchinenoside E1
Catalog No.:BCN8185
CAS No.:146100-02-9
[Analysis of lac color in diets and feces of rats for toxicity studies].[Pubmed:12092412]
Shokuhin Eiseigaku Zasshi. 2002 Apr;43(2):110-3.
An analytical method was developed for lac color in diets fed to rats and in the feces, and the contents of lac color were determined. After lac color was extracted with 0.05% sodium carbonate and 50% ethanol containing 0.02% sodium lauryl sulfate from the diets and feces, the extracted color solutions were analyzed by HPLC. The recoveries of lac color from diets spiked at 1.25, 5.00% and that from feces spiked at 5.00% were 85.6, 93.4% and 69.5%, respectively. Contents of lac color in diets prepared to contain 1.25 and 5.00% were 1.1 and 5.2%, and dose levels were confirmed by these results. Contents of lac color in feces of male and female rats given lac color were 127.8 mg/g and 138.6 mg/g, respectively. By comparing the HPLC chromatograms of laccaic acids in the diet with those in feces of rats, laccaic acid A, B, C and E were detected in both, and their content ratios were approximately determined.
Identification of anthraquinone coloring matters in natural red dyes by electrospray mass spectrometry coupled to capillary electrophoresis.[Pubmed:14696204]
J Mass Spectrom. 2003 Dec;38(12):1252-8.
Capillary electrophoresis with UV/visible diode-array detection (DAD) and electrospray mass spectrometric (ESI-MS) detection were used for the identification of anthraquinone color components of cochineal, lac-dye and madder, natural red dyestuffs often used by ancient painters. For the purpose of such analysis, ESI-MS was found to be a much more appropriate detection technique than DAD one owing to its higher sensitivity (detection limits in the range 0.1-0.5 micro g ml(-1)) and selectivity. The method developed made it possible to identify unequivocally carminic acid and laccaic acids A, B and E as coloring matters in the examined preparations of cochineal and lac-dye, respectively. In madder, European Rubia tinctorum, alizarin and purpurin were found. The method allows the rapid, direct and straightforward identification and quantification of components of natural products used in art and could be very helpful in restoration and conservation procedures.


