DorzolamideCAS# 120279-96-1 |
- Tigecycline mesylate
Catalog No.:BCC4229
CAS No.:1135871-27-0
- Amphotericin B
Catalog No.:BCN2564
CAS No.:1397-89-3
- Nystatin (Fungicidin)
Catalog No.:BCC4813
CAS No.:1400-61-9
- Tigecycline hydrochloride
Catalog No.:BCC4228
CAS No.:197654-04-9
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- Norfloxacin hydrochloride
Catalog No.:BCC4230
CAS No.:68077-27-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 120279-96-1 | SDF | Download SDF |
| PubChem ID | 5284549 | Appearance | Powder |
| Formula | C10H16N2O4S3 | M.Wt | 324.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | L671152; MK507 | ||
| Solubility | Soluble in DMSO | ||
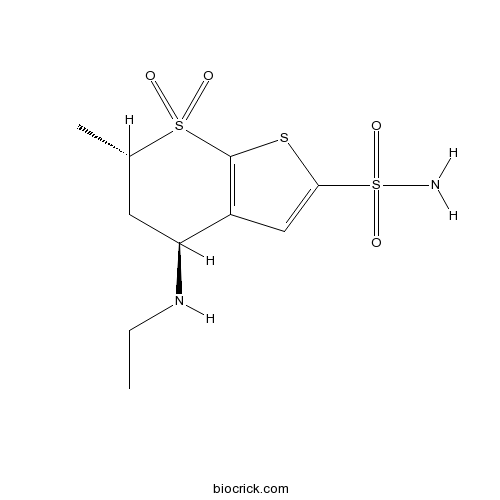
| Chemical Name | (4S,6S)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide | ||
| SMILES | CCNC1CC(S(=O)(=O)C2=C1C=C(S2)S(=O)(=O)N)C | ||
| Standard InChIKey | IAVUPMFITXYVAF-XPUUQOCRSA-N | ||
| Standard InChI | InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dorzolamide(L671152; MK507) is an anti-glaucoma agent, which is a carbonic anhydrase inhibitor.
Target: carbonic anhydrase (CA)
Dorzolamide is a carbonic anhydrase inhibitor. It is an anti-glaucoma agent, and acts by decreasing the production of aqueous humour [1].
Glaucoma was induced in the right eye of adult Wistar rats by episcleral venous occlusion. One experimental group was administered dorzolamide 2%-timolol 0.5% combination eye drops, while the other experimental group was administered dorzolamide 2% eye drops. Control groups had surgery without drug administration. Drug application was initiated either 2 weeks before surgery (Group A), from the day of surgery (Group B), 2 weeks after surgery (Group C), or 4 weeks after surgery (Group D). RGCs were labeled by intratectal Fluorogold injections and counted from flat-mount preparations, and IOP was measured using Tonopen. Both dorzolamide-timolol combination and dorzolamide, when applied topically, significantly reduced IOP and improved RGC densities in experimental eyes when compared to control eyes. Earlier initiation, as well as longer duration of drug application, resulted in higher RGC densities [2].
Clinical indications: Glaucoma; Ocular hypertension
FDA Approved Date: 1995
Toxicity: Dizziness, headache, shortness of breath, slow heartbeat, severe asthma, cardiac arrest References: | |||||

Dorzolamide Dilution Calculator

Dorzolamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0822 mL | 15.4112 mL | 30.8223 mL | 61.6447 mL | 77.0559 mL |
| 5 mM | 0.6164 mL | 3.0822 mL | 6.1645 mL | 12.3289 mL | 15.4112 mL |
| 10 mM | 0.3082 mL | 1.5411 mL | 3.0822 mL | 6.1645 mL | 7.7056 mL |
| 50 mM | 0.0616 mL | 0.3082 mL | 0.6164 mL | 1.2329 mL | 1.5411 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3082 mL | 0.6164 mL | 0.7706 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dorzolamide is an anti-glaucoma agent, which is a carbonic anhydrase inhibitor.
- 4-Hydroxysapriparaquinone
Catalog No.:BCN4806
CAS No.:120278-25-3
- Salvinolone
Catalog No.:BCN3215
CAS No.:120278-22-0
- CNX-774
Catalog No.:BCC4394
CAS No.:1202759-32-7
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- CGS 21680
Catalog No.:BCC1475
CAS No.:120225-54-9
- 1beta-Hydroxyeuscaphic acid
Catalog No.:BCN3517
CAS No.:120211-98-5
- Tenidap
Catalog No.:BCC7419
CAS No.:120210-48-2
- NMS-P715
Catalog No.:BCC6373
CAS No.:1202055-34-2
- Clopidogrel Related Compound C
Catalog No.:BCN2689
CAS No.:120202-71-3
- Clopidogrel
Catalog No.:BCC2497
CAS No.:120202-66-6
- 3,4-Dihydroxycinnamamide
Catalog No.:BCN6090
CAS No.:1202-41-1
- Cynoglossamine
Catalog No.:BCN1970
CAS No.:120193-39-7
- CX-6258
Catalog No.:BCC1504
CAS No.:1202916-90-2
- Citroside A
Catalog No.:BCN7294
CAS No.:120330-44-1
- DASA-58
Catalog No.:BCC6522
CAS No.:1203494-49-8
- Cyclotraxin B
Catalog No.:BCC6357
CAS No.:1203586-72-4
- AS 1949490
Catalog No.:BCC7762
CAS No.:1203680-76-5
- Jionoside B1
Catalog No.:BCN2858
CAS No.:120406-37-3
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- AZD1208
Catalog No.:BCC2079
CAS No.:1204144-28-4
- Icotinib Hydrochloride
Catalog No.:BCC1639
CAS No.:1204313-51-8
- TMC647055
Catalog No.:BCC6376
CAS No.:1204416-97-6
- Verlukast
Catalog No.:BCC2035
CAS No.:120443-16-5
- Jionoside A1
Catalog No.:BCN2922
CAS No.:120444-60-2
Additive effects and safety of fixed combination therapy with 1% brinzolamide and 0.5% timolol versus 1% dorzolamide and 0.5% timolol in prostaglandin-treated glaucoma patients.[Pubmed:28371482]
Acta Ophthalmol. 2017 Dec;95(8):e720-e726.
PURPOSE: To compare the additive effects and safety of 1% brinzolamide/0.5% timolol fixed combination (BTFC) versus the low-dose regimen of 1% Dorzolamide/0.5% timolol fixed combination (DTFC) in patients with open-angle glaucoma and ocular hypertension (OAG/OH) following treatment with prostaglandin analogues (PGAs). METHODS: A prospective, randomized, double-masked, multicentre, parallel-group and active-controlled study included 201 Japanese OAG/OH patients who had been treated with PGA. Efficacy was assessed as the change in intra-ocular pressure (IOP) from baseline after weeks 4 and 8. Safety was assessed with adverse event rates, ocular discomfort score, blur scale, blood pressure and heart rates, best-corrected visual acuity (BCVA) and slit lamp examinations. RESULTS: Intra-ocular pressure (IOP) change from baseline at 9 AM/11 AM pooled over the 8 weeks was -3.3/-3.3 mmHg in the BTFC group and -2.9/-3.4 mmHg in the DTFC group, demonstrating non-inferiority of BTFC to DTFC. Ocular irritation was frequently seen in DTFC group. Although blurred vision was frequently seen in BTFC group, it was transient and blurring became the equivalent 3 min after instillation between two groups. No noteworthy issue was observed in other safety outcome. CONCLUSION: Non-inferiority of BTFC to DTFC in IOP reduction was demonstrated after adding onto PGA therapy in Japanese OAG/OH patients. Although the score of blurred vision was transiently higher in BTFC than DTFC, treatment difference decreased and disappeared with time. Thus, BTFC can be considered as a safe and effective agent for glaucoma treatment.
Efficacy and safety of preoperative IOP reduction using a preservative-free fixed combination of dorzolamide/timolol eye drops versus oral acetazolamide and dexamethasone eye drops and assessment of the clinical outcome of trabeculectomy in glaucoma.[Pubmed:28199397]
PLoS One. 2017 Feb 15;12(2):e0171636.
INTRODUCTION: To demonstrate that preoperative treatment for 28 days with topical Dorzolamide/timolol is non-inferior (Delta = 4 mm Hg) to oral acetazolamide and topical dexamethasone (standard therapy) in terms of intraocular pressure (IOP) reduction 3 and 6 months after trabeculectomy in glaucoma patients. MATERIALS AND METHODS: Sixty-two eyes undergoing trabeculectomy with mitomycin C were included in this monocentric prospective randomized controlled study. IOP change between baseline and 3 months post-op was defined as the primary efficacy variable. Secondary efficacy variables included the number of 5-fluorouracil (5-FU) injections, needlings, suture lyses, preoperative IOP change, hypertension rate and change of conjunctival redness 3 and 6 months post-op. Safety was assessed based on the documentation of adverse events. RESULTS: Preoperative treatment with topical Dorzolamide/timolol was non-inferior to oral acetazolamide and topical dexamethasone in terms of IOP reduction 3 months after trabeculectomy (adjusted means -8.12 mmHg versus -8.30 mmHg; Difference: 0.18; 95% CI -1.91 to 2.26, p = 0.8662). Similar results were found 6 months after trabeculectomy (-9.13 mmHg versus -9.06 mmHg; p = 0.9401). Comparable results were also shown for both groups concerning the classification of the filtering bleb, corneal staining, and numbers of treatments with 5-FU, needlings and suture lyses. More patients reported AEs in the acetazolamide/dexamethasone group than in the Dorzolamide/timolol group. DISCUSSION: Preoperative, preservative-free, fixed-dose Dorzolamide/timolol seems to be equally effective as preoperative acetazolamide and dexamethasone and has a favourable safety profile.
New Electrochemically-Modified Carbon Paste Inclusion beta-Cyclodextrin and Carbon Nanotubes Sensors for Quantification of Dorzolamide Hydrochloride.[Pubmed:27918458]
Int J Mol Sci. 2016 Dec 2;17(12). pii: ijms17122027.
The present article introduces a new approach to fabricate carbon paste sensors, including carbon paste, modified carbon paste inclusion beta-cyclodextrin, and carbon nanotubes for the quantification of Dorzolamide hydrochloride (DRZ). This study is mainly based on the construction of three different carbon paste sensors by the incorporation of DRZ with phosphotungstic acid (PTA) to form Dorzolamide-phosphotungstate (DRZ-PT) as an electroactive material in the presence of the solvent mediator ortho-nitrophenyloctyl ether (o-NPOE). The fabricated conventional carbon paste sensor (sensor I), as well as the other modified carbon paste sensors using beta-cyclodextrin (sensor II) and carbon nanotubes (sensor III), have been investigated. The sensors displayed Nernstian responses of 55.4 +/- 0.6, 56.4 +/- 0.4 and 58.1 +/- 0.2 mV.decade(-1) over concentration ranges of 1.0 x 10(-5)-1.0 x 10(-2), 1.0 x 10(-6)-1.0 x 10(-2), and 5.0 x 10(-8)-1.0 x 10(-2) mol.L(-1) with lower detection limits of 5.0 x 10(-6), 5.0 x 10(-7), and 2.5 x 10(-9) mol.L(-1) for sensors I, II, and III, respectively. The critical performance of the developed sensors was checked with respect to the effect of various parameters, including pH, selectivity, response time, linear concentration relationship, lifespan, etc. Method validation was applied according to the international conference on harmonisation of technical requirements for registration of pharmaceuticals for human use ICH guidelines. The developed sensors were employed for the determination of DRZ in its bulk and dosage forms, as well as bio-samples. The observed data were statistically analyzed and compared with those obtained from other published methods.
Enhanced ocular efficacy of topically-delivered dorzolamide with nanostructured mucoadhesive microparticles.[Pubmed:28216468]
Int J Pharm. 2017 Apr 30;522(1-2):66-73.
Dorzolamide eye drops are widely prescribed to reduce intraocular pressure (IOP) in the treatment of ocular hypertension and glaucoma. However, in an eye drop formulation, Dorzolamide is rapidly cleared from the preocular space, hence requiring multiple daily administrations. Here, we sought to increase the preocular retention of Dorzolamide using nanostructured, mucoadhesive microparticles (MUCO_NM) as carriers for topical delivery to the eye. MUCO_NM were prepared by freeze-milling Dorzolamide-loaded, electrospun nanofibers composed of poly(lactic-co-glycolic acid) and polyethylene glycol. The microparticles were embedded in a rapidly-dissolving tablet of polyvinyl alcohol. To assess in vivo efficacy, the MUCO_NM were administered topically to the eyes of rabbits, and IOP was measured and compared to that in eyes treated with Trusopt((R)), a marketed eye drop of Dorzolamide. The MUCO_NM showed a 35% greater maximum IOP decrease and a>2-fold increase in the duration of the IOP decrease, compared to Trusopt((R)). This enhanced efficacy was comparable to that obtained with a single administration of 4 drops of Trusopt((R)) or 2 administrations of Trusopt((R)) at a 4-h interval. Our findings suggest that this MUCO_NM preparation is a promising carrier for topical delivery of Dorzolamide to the eye, with enhanced drug efficacy and the potential to reduce administration frequency.


