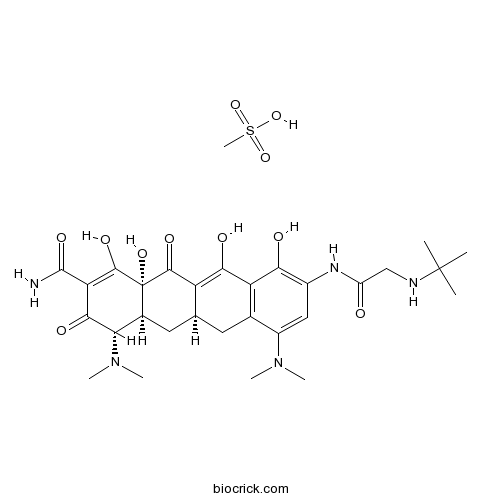
Tigecycline mesylateCAS# 1135871-27-0 |
- MGCD-265
Catalog No.:BCC2479
CAS No.:875337-44-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1135871-27-0 | SDF | Download SDF |
| PubChem ID | 78358330 | Appearance | Powder |
| Formula | C30H43N5O11S | M.Wt | 681.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GAR-936 mesylate | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | (4S,4aS,5aR,12aR)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide;methanesulfonic acid | ||
| SMILES | CC(C)(C)NCC(=O)NC1=C(C2=C(CC3CC4C(C(=O)C(=C(C4(C(=O)C3=C2O)O)O)C(=O)N)N(C)C)C(=C1)N(C)C)O.CS(=O)(=O)O | ||
| Standard InChIKey | QRMALYUMKBOEGR-KXLOKULZSA-N | ||
| Standard InChI | InChI=1S/C29H39N5O8.CH4O3S/c1-28(2,3)31-11-17(35)32-15-10-16(33(4)5)13-8-12-9-14-21(34(6)7)24(38)20(27(30)41)26(40)29(14,42)25(39)18(12)23(37)19(13)22(15)36;1-5(2,3)4/h10,12,14,21,31,36-37,40,42H,8-9,11H2,1-7H3,(H2,30,41)(H,32,35);1H3,(H,2,3,4)/t12-,14-,21-,29-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tigecycline mesylate a first-in-class, broad spectrum antibiotic with activity against antibiotic-resistant organisms.
Target: Antibacterial
Tigecycline mesylate is active against a broad range of gram-negative and gram-positive bacterial species including clinically important multidrug-resistant nosocomial and community-acquired bacterial pathogens. Tigecycline mesylate has been shown to inhibit the translation elongation step by binding to the ribosome 30S subunit and preventing aminoacylated tRNAs to accommodate in the ribosomal A site [1]. Tigecycline mesylate has also been found to be effective for the treatment of community- as well as hospital-acquired and ventilator-associated pneumonia and bacteremia, sepsis with shock and urinary tract infections. Tigecycline mesylate appears to be a valuable treatment option for the management of superbugs, especially where conventional therapy has failed [2].
Fifteen patients received tigecycline mesylate for 16 episodes of CPKP infection. The main infections were pneumonia (31%), urinary tract infection (31%), peritonitis (20%), catheter-related bacteraemia (12%), and meningitis (6%). Most infections were complicated with severe sepsis (44%), septic shock (12%), and/or bacteraemia (19%). The daily maintenance dose of tigecycline mesylate was 200 mg in 10 episodes and 100 mg in 6 episodes. The overall 30-day mortality rate was 25%. Univariate analysis showed that mortality was significantly associated (p < 0.01) with mean APACHE II and SOFA scores and the presence of immunosuppression, but not with the tigecycline mesylate dose [3].
Clinical indications: Acinetobacter infection; Bacterial infection; Bacterial pneumonia; Bacterial skin infection; Bacteroides fragilis infection; Bacteroides infection; Citrobacter infection; Clostridiaceae infection; Clostridium difficile infection; Clostridium infection; Enterobacter infection
FDA Approved Date: June 17, 2005
Toxicity: nausea; vomiting; diarrhea; local IV-site reaction; infection; fever; headache References: | |||||

Tigecycline mesylate Dilution Calculator

Tigecycline mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4668 mL | 7.3341 mL | 14.6681 mL | 29.3363 mL | 36.6703 mL |
| 5 mM | 0.2934 mL | 1.4668 mL | 2.9336 mL | 5.8673 mL | 7.3341 mL |
| 10 mM | 0.1467 mL | 0.7334 mL | 1.4668 mL | 2.9336 mL | 3.667 mL |
| 50 mM | 0.0293 mL | 0.1467 mL | 0.2934 mL | 0.5867 mL | 0.7334 mL |
| 100 mM | 0.0147 mL | 0.0733 mL | 0.1467 mL | 0.2934 mL | 0.3667 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tigecycline mesylate a first-in-class, broad spectrum antibiotic with activity against antibiotic-resistant organisms.
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- E-4031 dihydrochloride
Catalog No.:BCC7182
CAS No.:113559-13-0
- Baohuoside I
Catalog No.:BCN5350
CAS No.:113558-15-9
- Ikarisoside F
Catalog No.:BCN2284
CAS No.:113558-14-8
- 1,2,3,19-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1615
CAS No.:113558-03-5
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- Orbifloxacin
Catalog No.:BCC4689
CAS No.:113617-63-3
- 6beta-(Hexa-2,4-dienoyloxy)-9alpha,12-dihydroxydrimenol
Catalog No.:BCN7277
CAS No.:1136245-81-2
- Metasequoic acid A
Catalog No.:BCN6652
CAS No.:113626-22-5
- Stigmast-4-ene-3,6-diol
Catalog No.:BCN6014
CAS No.:113626-76-9
- IDE 2
Catalog No.:BCC6099
CAS No.:1136466-93-7
- Ustusolate A
Catalog No.:BCN6756
CAS No.:1136611-58-9
- Neuropeptide Y 13-36 (porcine)
Catalog No.:BCC6959
CAS No.:113662-54-7
- 3-(hydroxymethyl)cyclopentanone
Catalog No.:BCN6015
CAS No.:113681-11-1
- Shizukanolide H
Catalog No.:BCN6016
CAS No.:1136932-34-7
- 4-Aminobenzophenone
Catalog No.:BCC8684
CAS No.:1137-41-3
- BOC-D-ARG-OH.HCL.H2O
Catalog No.:BCC3069
CAS No.:113712-06-4
- Tenatoprazole
Catalog No.:BCC4732
CAS No.:113712-98-4
Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells.[Pubmed:28920959]
Nat Med. 2017 Oct;23(10):1234-1240.
Treatment of chronic myeloid leukemia (CML) with imatinib mesylate and other second- and/or third-generation c-Abl-specific tyrosine kinase inhibitors (TKIs) has substantially extended patient survival. However, TKIs primarily target differentiated cells and do not eliminate leukemic stem cells (LSCs). Therefore, targeting minimal residual disease to prevent acquired resistance and/or disease relapse requires identification of new LSC-selective target(s) that can be exploited therapeutically. Considering that malignant transformation involves cellular metabolic changes, which may in turn render the transformed cells susceptible to specific assaults in a selective manner, we searched for such vulnerabilities in CML LSCs. We performed metabolic analyses on both stem cell-enriched (CD34(+) and CD34(+)CD38(-)) and differentiated (CD34(-)) cells derived from individuals with CML, and we compared the signature of these cells with that of their normal counterparts. Through combination of stable isotope-assisted metabolomics with functional assays, we demonstrate that primitive CML cells rely on upregulated oxidative metabolism for their survival. We also show that combination treatment with imatinib and tigecycline, an antibiotic that inhibits mitochondrial protein translation, selectively eradicates CML LSCs both in vitro and in a xenotransplantation model of human CML. Our findings provide a strong rationale for investigation of the use of TKIs in combination with tigecycline to treat patients with CML with minimal residual disease.
Gateways to clinical trials.[Pubmed:19536362]
Methods Find Exp Clin Pharmacol. 2009 Apr;31(3):183-226.
(+)-Dapoxetine hydrochloride, [(123)I]-BZA, 9-Aminocamptothecin; Abacavir sulfate/lamivudine, Adalimumab, Adefovir dipivoxil, Alemtuzumab, Alvocidib hydrochloride, Ambrisentan, Amsilarotene, Anacetrapib, Anakinra, Apricitabine, Aripiprazole, Arsenic trioxide, Atazanavir sulfate, Atazanavir/ritonavir, Atrasentan, Azacitidine; Banoxantrone, Bazedoxifene acetate, Bevacizumab, Bexarotene, Biphasic insulin aspart, Bortezomib, Bosentan, Bromfenac; Cachectin, Calcipotriol/betamethasone dipropionate, Canakinumab, Carfilzomib, CAT-354, CCX-282, Certolizumab pegol, Cetuximab, Choline fenofibrate, Clevudine, Clofarabine, CNTO-328, Corifollitropin alfa, Crofelemer; Daptomycin, Darbepoetin alfa, Darunavir, Dasatinib, Decitabine, Deferasirox, Denosumab, Duloxetine hydrochloride, Dutasteride; Emtricitabine, Enfuvirtide, Entecavir, Epoetin zeta, Erlotinib hydrochloride, Escitalopram oxalate, Eslicarbazepine acetate, Eszopiclone, Etravirine, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; Farglitazar, Febuxostat, Fosamprenavir calcium, FX-06; Gabapentin enacarbil, Gefitinib; HIVIS DNA; Imatinib mesylate, INCB- 18424, Indacaterol, Inotuzumab ozogamicin, Insulin detemir; JNJ-26854165; Lacosamide, Landiolol, Laromustine, Lenalidomide, Liposomal doxorubicin, L-NAME, Lopinavir, Lopinavir/ritonavir, Lumiracoxib; Maraviroc, Mepolizumab, Methoxy polyethylene glycol- epoetin-beta, Miglustat, MK-0493, MVA-CMDR, Mycophenolic acid sodium salt; Natalizumab, Nepafenac, Neratinib, Neridronic acid, Nesiritide, Nilotinib hydrochloride monohydrate; Olmesartan medoxomil, Omacetaxine mepesuccinate, Omalizumab; Paclitaxel poliglumex, Palifermin, Patupilone, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b, Peginterferon alfa-2b/ ribavirin, Pemetrexed disodium, PHA-848125, Pitavastatin calcium, Posaconazole, Povidone-iodine liposome complex, Prasugrel, Pregabalin, Prucalopride; Raltegravir potassium, Retigabine, Revaprazan hydrochloride, rhFSH, Rilpivirine, Rivaroxaban, Romidepsin, Rosuvastatin calcium, RWJ-676070; SAR-109659, Sitagliptin phosphate monohydrate, Sorafenib, Stavudine/Lamivudine/Nevirapine, Sunitinib malate; Tadalafil, Telaprevir, Telbivudine, Tenofovir disoproxil fumarate, Tenofovir disoproxil fumarate/emtricitabine, Tenofovir disoproxil fumarate/emtricitabine/efavirenz, Teriparatide, Tigecycline, Tiotropium bromide, Tipifarnib, Tipranavir, Tocilizumab, Trifluridine/TPI; UP-780; Vandetanib, Vardenafil hydrochloride hydrate, Vatalanib succinate, Vitespen, Vorinostat; Yttrium 90 (90Y) ibritumomab tiuxetan; Zoledronic acid monohydrate.
Gateways to clinical trials.[Pubmed:21225012]
Methods Find Exp Clin Pharmacol. 2010 Dec;32(10):749-73.
Gateways to Clinical Trials is a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Thomson Reuters Integrity(SM), the drug discovery and development portal, http://www.thomsonreutersintegrity.com. This issue focuses on the following selection of drugs: 17-Hydroxyprogesterone caproate; Abacavir sulfate/lamivudine, Aclidinium bromide, Adalimumab, Adefovir, Alemtuzumab, Alkaline phosphatase, Amlodipine, Apilimod mesylate, Aripiprazole, Axitinib, Azacitidine; Belotecan hydrochloride, Berberine iodide, Bevacizumab, Bortezomib, Bosentan, Bryostatin 1; Calcipotriol/hydrocortisone, Carglumic acid, Certolizumab pegol, Cetuximab, Cinacalcet hydrochloride, Cixutumumab, Coumarin, Custirsen sodium; Darbepoetin alfa, Darifenacin hydrobromide, Darunavir, Dasatinib, Denibulin hydrochloride, Denosumab, Diacetylmorphine, Dulanermin, Duloxetine hydrochloride; Ecogramostim, Enfuvirtide, Entecavir, Enzastaurin hydrochloride, Eplerenone, Escitalopram oxalate, Esomeprazole sodium, Etravirine, Everolimus, Ezetimibe; Fenofibrate/pravastatin sodium, Ferric carboxymaltose, Flavangenol, Fondaparinux sodium; Glutamine, GSK-1024850A; Hepatitis B hyperimmunoglobulin, Hib-MenC, HIV-LIPO-5; Immunoglobulin intravenous (human), Indacaterol maleate, Indibulin, Indium 111 ((1)(1)(1)In) ibritumomab tiuxetan, Influenza A (H1N1) 2009 Monovalent vaccine, Inhalable human insulin, Insulin glulisine; Lapatinib ditosylate, Leucovorin/UFT; Maraviroc, Mecasermin, MMR-V, Morphine hydrochloride, Morphine sulfate/naltrexone hydrochloride, Mycophenolic acid sodium salt; Naproxen/esomeprazole magnesium, Natalizumab; Oncolytic HSV; Paliperidone, PAN-811, Paroxetine, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b/ribavirin, Pegvisomant, Pemetrexed disodium, Pimecrolimus, Posaconazole, Pregabalin; Raltegravir potassium, Ranelic acid distrontium salt, Rasburicase, Rilpivirine hydrochloride; Sertindole, Sivelestat sodium hydrate, Sorafenib, Sumatriptan succinate/naproxen sodium, Sunitinib malate; Tafluprost, Telithromycin, Temsirolimus, Tenofovir disoproxil fumavate, Tenofovir disoproxil fumarate/emtricitabine, Teriparatide, Ticagrelor, Tigecycline, Tipranavir, Tirapazamine, Trimetrexate; Ulipristal acetate; Valganciclovir hydrochloride, Vicriviroc, Vorinostat; Yttrium 90 (90Y) ibritumomab tiuxetan.
Gateways to clinical trials.[Pubmed:20094643]
Methods Find Exp Clin Pharmacol. 2009 Nov;31(9):597-633.
Abacavir sulfate/lamivudine, Adalimumab, AdCD40L, Adefovir, Adefovir dipivoxil, Ambrisentan, Amlodipine, Amlodipine besylate/olmesartan medoxomil, AN-2728, Apixaban, Aripiprazole, Armodafinil, Atazanavir sulfate, Atomoxetine hydrochloride, Atrasentan, Azacitidine, Bevacizumab, Blinatumomab, Bortezomib, Bosentan, Carfilzomib, Caspofungin acetate, Cediranib, Cetuximab, Choriogonadotropin alfa, Clevudine, Clindamycin phosphate/benzoyl peroxide, Clofarabine, Daidzeol, Darunavir, Dasatinib, Decitabine, Deferasirox, Deforolimus, Degarelix acetate, Denenicokin, Dexlansoprazole, Duloxetine hydrochloride, Elacytarabine, Enfuvirtide, Enoxaparin, Entecavir, Eribulin mesilate, Erlotinib hydrochloride, Escitalopram oxalate, Eslicarbazepine acetate, Eszopiclone, Etravirine, Ezetimibe/simvastatin, Forodesine hydrochloride, Fosamprenavir calcium, Gefitinib, Gemtuzumab ozogamicin, Golimumab, Imatinib mesylate, Imetelstat, Insulin gl'argine, Insulin glulisine, Interferon alfa-2b XL, Ivabradine hydrochloride, Lacosamide, Lenalidomide, Lintuzumab, Liposomal adriamycin, Liposomal belotecan, Liposome-encapsulated fentanyl, Lopinavir/ritonavir, Lutropin alfa, LY-207320, Maraviroc, Mecasermin, MKC-253, MP-470, NGR-TNF, Nilotinib hydrochloride monohydrate, Ofatumumab, Olmesartan medoxomil, Omacetaxine mepesuccinate, PAN-811, Panobinostat, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b, Peginterferon alfa-2b/ribavirin, Pemetrexed disodium, Perospirone hydrochloride, PF-734200, Phentermine/topiramate, Pimecrolimus, Pitavastatin calcium, Plerixafor hydrochloride, Pregabalin, Raltegravir potassium, Ramelteon, Ranibizumab, Recombinant Bet V1, Recombinant human insulin, Regadenoson, rhITF, Romidepsin, Rosuvastatin calcium, Ruboxistaurin hydrochloride, Rufinamide, Sapropterin dihydrochloride Saracatinib, SB-73, SC-599, Seliciclib, Sirolimus-eluting stent, Sorafenib, Sunitinib malate, Tadalafil, Tanespimycin, Tapentadol hydrochloride, Tegaserod maleate, Telbivudine, Tenofovir disoproxil fumarate, Tenofovir disoproxil fumarate/emtricitabine, Tenofovir disoproxil fumarate/emtricitabine/efavirenz, Ticlopidine hydrochloride, Tigecycline, TST-10088, Tularemia vaccine, Valsartan/amlodipine besylate, Vandetanib, Vardenafil hydrochloride hydrate, Vincristine, Vorinostat, Yttrium 90 (90Y) ibritumomab tiuxetan.
Gateways to clinical trials.[Pubmed:20664824]
Methods Find Exp Clin Pharmacol. 2010 Jun;32(5):331-88.
[(1)(1)C]RAC; (18)F-Fluoromisonidazole; 89-12; 9-[(1)(8)F]Fluoropropyl-(+)-dihydrotetrabenazine; Adalimumab, Adecatumumab, ADMVA, ADXS-11-001, Aflibercept, Agatolimod sodium, AGS-004, Alglucosidase alfa, Aliskiren fumarate, Alvocidib hydrochloride, AMG-108, AMG-853, Apixaban, Aripiprazole, Armodafinil, Atazanavir sulfate, Atomoxetine hydrochloride; Bevacizumab, BioMatrix Flex drug eluting stent, Biphasic insulin aspart, Bortezomib, Bosentan; Caspofungin acetate, Cediranib, Cetuximab, ChimeriVax-Dengue, Choriogonadotropin alfa, Cinacalcet hydrochloride, Cizolirtine citrate, Clofarabine, Cocaine conjugate vaccine, CX-717; Darbepoetin alfa, Dasatinib, Decitabine, Denosumab, Desvenlafaxine succinate, Dexamethasone sodium phosphate, Dienogest, Diphencyprone, Doripenem, DTaP-HepB-IPV, Dutasteride; E-7010, Ecallantide, Ecstasy, Eicosapentaenoic acid/docosahexaenoic acid, Emtricitabine, Enfuvirtide, Erlotinib hydrochloride, Eszopiclone, Etonogestrel/ethinyl estradiol, Etoricoxib, Everolimus, Everolimus-eluting coronary stent EVT-201, Ezetimibe, Ezetimibe/simvastatin; Ferumoxytol, Fesoterodine fumavate, Figitumumab, Filgrastim, Fingolimod hydrochloride, Fluticasone furoate, Fluval P, Fluzone, Fondaparinux sodium, Fulvestrant, Fungichromin; Gamma-hydroxybutyrate sodium, Gefitinib, GHB-01L1, GLY-230, GSK-1349572; Hib-MenCY-TT, Hib-TT, HPV-6/11/16/18, Hydrocodone bitartrate; IC-51, Icatibant acetate, Imatinib mesylate, Immunoglobulin intravenous (human), Indetanib, Influenza A (H1N1) 2009 Monovalent Vaccine, Inhalable human insulin, Insulin glargine, Insulin glulisine, Interferon-beta, Ispinesib mesylate, Ixabepilone; Laromustine, Latanoprost/timolol maleate, L-Citrulline, Lenalidomide, Lexatumumab, Linezolid, Lopinavir/ritonavir, Lutropin alfa; Mapatumumab, MDX-066, MDX-1388, Mepolizumab, Methoxy polyethylene glycol-epoetin-beta, Metreleptin, Micafungin sodium, Mometasone furoate/oxymetazoline hydrochloride, Mx-dnG1, Mycophenolic acid sodium salt; Nabiximols, Natalizumab, Nemonoxacin, Norelgestromin/ethinyl estradiol; Oblimersen sodium, Ocriplasmin, Olmesartan medoxomil, Omacetaxine mepesuccinate; Paclitaxel-eluting stent, Pagoclone, Paliperidone, Panitumumab, Pazopanib hydrochloride, PCV7, Pegaptanib octasodium, Peginterferon alfa-2a, Peginterferon alfa-2b/ ribavirin, Pegvisomant, Pemetrexed disodium, Perifosine, Pimecrolimus, Pitavastatin calcium, Plerixafor hydrochloride, Plitidepsin, Posaconazole, Pregabalin, Progesterone capriate; Raltegravir potassium, Ramucirumab, Ranelic acid distrontium salt, Rasburicase, Recombinant Bet V1, Recombinant human insulin, rhFSH, Rolofylline, Romidepsin, Romiplostim, Rosuvastatin calcium; Sapacitabine, Sevelamer carbonate, Sinecatechins, Sirolimus-eluting stent, Sitagliptin phosphate monohydrate, SN-29244, Sorafenib, Sugammadex sodium, Sunitinib malate; Tadalafil, Tafenoquine, Talnetant, Tanezumab, Tapentadol hydrochloride, Tasocitinib citrate, Technosphere/Insulin, Telcagepant, Tenofovir disoproxil fumarate, Teriparatide, Ticagrelor, Tigecycline, Tiotropium bromide, Tipifarnib, Tocilizumab, TS-041; Ulipristal acetate, Urtoxazumab, Ustekinumab; Vandetanib, Varenicline tartrate, Vicriviroc, Voriconazole, Vorinostat, VRC-HIVADV014-00-VP, VRC-HIVDNA016-00-VP; Zoledronic acid monohydrate.


