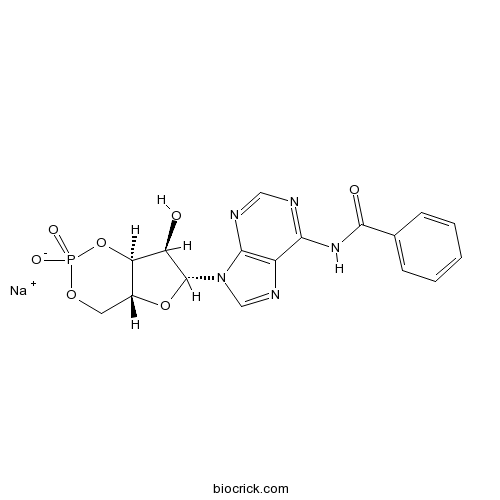
6-Bnz-cAMP sodium saltcAMP analog,PKA activator CAS# 1135306-29-4 |
- Edoxaban tosylate monohydrate
Catalog No.:BCC1545
CAS No.:1229194-11-9
- Otamixaban
Catalog No.:BCC1827
CAS No.:193153-04-7
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Rivaroxaban
Catalog No.:BCC2292
CAS No.:366789-02-8
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1135306-29-4 | SDF | Download SDF |
| PubChem ID | 23672707 | Appearance | Powder |
| Formula | C17H15N5NaO7P | M.Wt | 455.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | sodium;N-[9-[(4aR,6R,7R,7aS)-7-hydroxy-2-oxido-2-oxo-4a,6,7,7a-tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-6-yl]purin-6-yl]benzamide | ||
| SMILES | C1C2C(C(C(O2)N3C=NC4=C3N=CN=C4NC(=O)C5=CC=CC=C5)O)OP(=O)(O1)[O-].[Na+] | ||
| Standard InChIKey | SPYGSKQRPXISIB-FKVBDRBCSA-M | ||
| Standard InChI | InChI=1S/C17H16N5O7P.Na/c23-12-13-10(6-27-30(25,26)29-13)28-17(12)22-8-20-11-14(18-7-19-15(11)22)21-16(24)9-4-2-1-3-5-9;/h1-5,7-8,10,12-13,17,23H,6H2,(H,25,26)(H,18,19,21,24);/q;+1/p-1/t10-,12-,13-,17-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell permeable cAMP analog; selectively activates cAMP-dependent PKA but not Epac signaling pathways. Acts synergistically with 8-CPT-2Me-cAMP to inhibit vascular smooth muscle cell proliferation. |

6-Bnz-cAMP sodium salt Dilution Calculator

6-Bnz-cAMP sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1964 mL | 10.982 mL | 21.964 mL | 43.928 mL | 54.9101 mL |
| 5 mM | 0.4393 mL | 2.1964 mL | 4.3928 mL | 8.7856 mL | 10.982 mL |
| 10 mM | 0.2196 mL | 1.0982 mL | 2.1964 mL | 4.3928 mL | 5.491 mL |
| 50 mM | 0.0439 mL | 0.2196 mL | 0.4393 mL | 0.8786 mL | 1.0982 mL |
| 100 mM | 0.022 mL | 0.1098 mL | 0.2196 mL | 0.4393 mL | 0.5491 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
6-Bnz-cAMP is a PKA-selective activator. It regulates the PKA dependent signaling pathways.
Like PKA contains an evolutionally conserved cAMP-binding domain that acts as a molecular switch for sensing intracellular second messenger cAMP levels to control diverse biological functions. CAMP response element-binding protein (CREB) is the well-known direct target protein of PKA. Phosphorylation of CREB (pCREB) by PKA has been shown to be involved in regulating osteoblast differentiation.
The proliferative signaling pathway which activated by the 6-Bnz-cAMP involves activation of the epidermal growth factor receptor and ERK1/2. Extending the duration of PKA-dependent ERK1/2 activation and converted cAMP from a proliferative into an anti-proliferative, neurite outgrowth- promoting signal.
6-Bnz-cAMP can promote not only differentiation and mineralization, but also initial cell adhesion.6-Bnz-cAMP is able to induce osteogenic differentiation of MC3T3-E1 cells. Moreover 6-Bnz-cAMP may facilitate release kinetic from a tissue-engineered polymeric scaffold system. It also can serve as a novel bone-inducing growth factor for repairing and regenerating bone tissues during bone regenerative engineering.
References:
[1]Lo KW, Kan HM, Ashe KM, Laurencin CT. The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. J Tissue Eng Regen Med. 2012 Jan;6(1):40-8.
[2]Simone Kiermayer, Ricardo M. Biondi,etal., Epac Activation Converts cAMP from a Proliferative into a Differentiation Signal in PC12 Cells. Molecular Biology of the Cell. Vol. 16, 5639–5648, December 2005.
[3]Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai). 2008 Jul;40(7):651-62.
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- CGP 78608 hydrochloride
Catalog No.:BCC7087
CAS No.:1135278-54-4
- SR 59230A hydrochloride
Catalog No.:BCC7094
CAS No.:1135278-41-9
- VU 0255035
Catalog No.:BCC7766
CAS No.:1135243-19-4
- VU 0357017 hydrochloride
Catalog No.:BCC7907
CAS No.:1135242-13-5
- Tracazolate hydrochloride
Catalog No.:BCC7115
CAS No.:1135210-68-2
- Moxidectin
Catalog No.:BCC5309
CAS No.:113507-06-5
- Ferulic acid
Catalog No.:BCN5948
CAS No.:1135-24-6
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
- 1,2,3,19-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1615
CAS No.:113558-03-5
- Ikarisoside F
Catalog No.:BCN2284
CAS No.:113558-14-8
- Baohuoside I
Catalog No.:BCN5350
CAS No.:113558-15-9
- E-4031 dihydrochloride
Catalog No.:BCC7182
CAS No.:113559-13-0
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- Tigecycline mesylate
Catalog No.:BCC4229
CAS No.:1135871-27-0
- Orbifloxacin
Catalog No.:BCC4689
CAS No.:113617-63-3
- 6beta-(Hexa-2,4-dienoyloxy)-9alpha,12-dihydroxydrimenol
Catalog No.:BCN7277
CAS No.:1136245-81-2
- Metasequoic acid A
Catalog No.:BCN6652
CAS No.:113626-22-5
Salt Sensitivity of Angiogenesis Inhibition-Induced Blood Pressure Rise: Role of Interstitial Sodium Accumulation?[Pubmed:28320855]
Hypertension. 2017 May;69(5):919-926.
In response to salt loading, Na(+) and Cl(-) accumulate in the skin in excess of water, stimulating skin lymphangiogenesis via activation of the mononuclear phagocyte system cell-derived vascular endothelial growth factor-C-vascular endothelial growth factor type 3 receptor signaling pathway. Inhibition of this pathway results in salt-sensitive hypertension. Sunitinib is an antiangiogenic, anticancer agent that blocks all 3 vascular endothelial growth factor receptors and increases blood pressure. We explored the salt dependency of sunitinib-induced hypertension and whether impairment of skin lymphangiogenesis is an underlying mechanism. Normotensive Wistar-Kyoto rats were exposed to a normal or high salt with or without sunitinib administration. Sunitinib induced a 15 mm Hg rise in telemetrically measured blood pressure, which was aggravated by a high-salt diet (HSD), resulting in a decline of the slope of the pressure-natriuresis curve. Without affecting body weight, plasma Na(+) concentration or renal function, Na(+) and Cl(-) skin content increased by 31% and 32% with the high salt and by 49% and 50% with the HSD plus sunitinib, whereas skin water increased by 17% and 24%, respectively. Skin mononuclear phagocyte system cell density increased both during sunitinib and a HSD, but no further increment was seen when HSD and sunitinib were combined. HSD increased skin lymphangiogenesis, while sunitinib tended to decrease lymphangiogenesis, both during a normal-salt diet and HSD. We conclude that sunitinib induces hypertension that is aggravated by high salt intake and not accompanied by impaired skin lymphangiogenesis.
A novel class of apical sodium--dependent bile salt transporter inhibitors: 1-(2,4-bifluorophenyl)-7-dialkylamino-1,8-naphthyridine-3-carboxamides.[Pubmed:28303230]
Acta Pharm Sin B. 2017 Mar;7(2):223-229.
The apical sodium--dependent bile acid transporter (ASBT) is the main transporter to promote re-absorption of bile acids from the intestinal tract into the enterohepatic circulation. Inhibition of ASBT could increase the excretion of bile acids, thus increasing bile acid synthesis and consequently cholesterol consumption. Therefore, ASBT is an attractive target for developing new cholesterol-lowering drugs. In this report, a series of 1-(2,4-bifluorophenyl)-7-dialkylamino-1,8-naphthyridine-3-carboxamides were designed as inhibitors of ASBT. Most of them demonstrated potency against ASBT transport of bile acids. In particular, compound 4a1 was found to have the best activity, resulting in 80.1% inhibition of ASBT at 10 mumol/L.
Sea salt sodium record from Talos Dome (East Antarctica) as a potential proxy of the Antarctic past sea ice extent.[Pubmed:28314231]
Chemosphere. 2017 Jun;177:266-274.
Antarctic sea ice has shown an increasing trend in recent decades, but with strong regional differences from one sector to another of the Southern Ocean. The Ross Sea and the Indian sectors have seen an increase in sea ice during the satellite era (1979 onwards). Here we present a record of ssNa(+) flux in the Talos Dome region during a 25-year period spanning from 1979 to 2003, showing that this marker could be used as a potential proxy for reconstructing the sea ice extent in the Ross Sea and Western Pacific Ocean at least for recent decades. After finding a positive relationship between the maxima in sea ice extent for a 25-year period, we used this relationship in the TALDICE record in order to reconstruct the sea ice conditions over the 20th century. Our tentative reconstruction highlighted a decline in the sea ice extent (SIE) starting in the 1950s and pointed out a higher variability of SIE starting from the 1960s and that the largest sea ice extents of the last century occurred during the 1990s.
Enantiomers of triclabendazole sulfoxide: Analytical and semipreparative HPLC separation, absolute configuration assignment, and transformation into sodium salt.[Pubmed:28340473]
J Pharm Biomed Anal. 2017 Jun 5;140:38-44.
Direct HPLC separation of the enantiomers of triclabendazole sulfoxide (TCBZ-SO), which is the main metabolite of the anthelmintic drug triclabendazole, was carried out using the polysaccharide-based Chiralpak AS-H and Chiralpak IF-3 chiral stationary phases (CSPs). The chromatographic behaviour of both CSPs was evaluated and compared using normal-phase and reversed-phase eluents at different column temperatures. The eluent mixture of n-hexane-2-propanol-trifluoroacetic acid 70:30:0.1 (v/v/v) and a column temperature of 40 degrees C were identified as the best operational conditions to carry out semipreparative enantioseparations on a 1-cm I.D. AS-H column. Under these conditions, 12.5mg of racemic sample were resolved in a single chromatographic run within 15min. Comparison of calculated and experimental chiroptical properties provided the absolute configuration assignment at the sulfur atom. The salification of the isolated enantiomers of TCBZ-SO by reaction with sodium hydroxide solution produced water-soluble Na salts which are potentially useful in the development of new anthelmintic enantiomerically pure formulations.
PKA and Epac synergistically inhibit smooth muscle cell proliferation.[Pubmed:20971121]
J Mol Cell Cardiol. 2011 Jan;50(1):87-98.
Cyclic AMP signalling promotes VSMC quiescence in healthy vessels and during vascular healing following injury. Cyclic AMP inhibits VSMC proliferation via mechanisms that are not fully understood. We investigated the role of PKA and Epac signalling on cAMP-induced inhibition of VSMC proliferation. cAMP-mediated growth arrest was PKA-dependent. However, selective PKA activation with 6-Benzoyl-cAMP did not inhibit VSMC proliferation, indicating a requirement for additional pathways. Epac activation using the selective cAMP analogue 8-CPT-2'-O-Me-cAMP, did not affect levels of hyperphosphorylated Retinoblastoma (Rb) protein, a marker of G1-S phase transition, or BrdU incorporation, despite activation of the Epac-effector Rap1. However, 6-Benzoyl-cAMP and 8-CPT-2'-O-Me-cAMP acted synergistically to inhibit Rb-hyperphosphorylation and BrdU incorporation, indicating that both pathways are required for growth inhibition. Consistent with this, constitutively active Epac increased Rap1 activity and synergised with 6-Benzoyl-cAMP to inhibit VSMC proliferation. PKA and Epac synergised to inhibit phosphorylation of ERK and JNK. Induction of stellate morphology, previously associated with cAMP-mediated growth arrest, was also dependent on activation of both PKA and Epac. Rap1 inhibition with Rap1GAP or siRNA silencing did not negate forskolin-induced inhibition of Rb-hyperphosphorylation, BrdU incorporation or stellate morphology. This data demonstrates for the first time that Epac synergises with PKA via a Rap1-independent mechanism to mediate cAMP-induced growth arrest in VSMC. This work highlights the role of Epac as a major player in cAMP-dependent growth arrest in VSMC.


