KN-92 phosphateCaMKII inhibitor CAS# 1135280-28-2 |
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- A 438079 hydrochloride
Catalog No.:BCC1317
CAS No.:899431-18-6
- A 438079
Catalog No.:BCC1316
CAS No.:899507-36-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1135280-28-2 | SDF | Download SDF |
| PubChem ID | 16219540 | Appearance | Powder |
| Formula | C24H28ClN2O7PS | M.Wt | 554.98 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (180.19 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
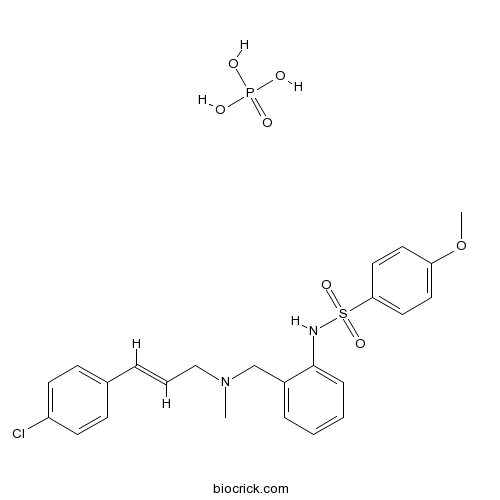
| Chemical Name | N-[2-[[[(E)-3-(4-chlorophenyl)prop-2-enyl]-methylamino]methyl]phenyl]-4-methoxybenzenesulfonamide;phosphoric acid | ||
| SMILES | CN(CC=CC1=CC=C(C=C1)Cl)CC2=CC=CC=C2NS(=O)(=O)C3=CC=C(C=C3)OC.OP(=O)(O)O | ||
| Standard InChIKey | XRQHWVVDNMJDEQ-IPZCTEOASA-N | ||
| Standard InChI | InChI=1S/C24H25ClN2O3S.H3O4P/c1-27(17-5-6-19-9-11-21(25)12-10-19)18-20-7-3-4-8-24(20)26-31(28,29)23-15-13-22(30-2)14-16-23;1-5(2,3)4/h3-16,26H,17-18H2,1-2H3;(H3,1,2,3,4)/b6-5+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inactive analog of the CaM kinase II inhibitor KN 93. Potassium channel (Kv1.2, 1.4, 1.5, 2.1, 3.2 and hERG) blocker in vitro. |

KN-92 phosphate Dilution Calculator

KN-92 phosphate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8019 mL | 9.0093 mL | 18.0187 mL | 36.0373 mL | 45.0467 mL |
| 5 mM | 0.3604 mL | 1.8019 mL | 3.6037 mL | 7.2075 mL | 9.0093 mL |
| 10 mM | 0.1802 mL | 0.9009 mL | 1.8019 mL | 3.6037 mL | 4.5047 mL |
| 50 mM | 0.036 mL | 0.1802 mL | 0.3604 mL | 0.7207 mL | 0.9009 mL |
| 100 mM | 0.018 mL | 0.0901 mL | 0.1802 mL | 0.3604 mL | 0.4505 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
KN-92 is an inactive derivative of KN-93. It is intended to be used as a control compound in studies designed to elucidate the antagonist activities of KN-93. KN-93 is a selective inhibitor of Ca2+/calmodulin-dependent kinase II (CaMKII), competitively blocking CaM binding to the kinase (Ki = 370 nM). KN-93 inhibits histamine-induced aminopyrine uptake in parietal cells (IC50 = 300 nM). KN-93 has been used to implicate roles for CaMKII in Ca2+-induced Ca2+ release in cardiac myocytes, constitutive phosphorylation of 5-lipoxygenase in 3T3 cells, and Ca2+-dependent activation of HIF-1α in colon cancer cell.
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- CGP 78608 hydrochloride
Catalog No.:BCC7087
CAS No.:1135278-54-4
- SR 59230A hydrochloride
Catalog No.:BCC7094
CAS No.:1135278-41-9
- VU 0255035
Catalog No.:BCC7766
CAS No.:1135243-19-4
- VU 0357017 hydrochloride
Catalog No.:BCC7907
CAS No.:1135242-13-5
- Tracazolate hydrochloride
Catalog No.:BCC7115
CAS No.:1135210-68-2
- Moxidectin
Catalog No.:BCC5309
CAS No.:113507-06-5
- Ferulic acid
Catalog No.:BCN5948
CAS No.:1135-24-6
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- Epidanshenspiroketallactone
Catalog No.:BCN3142
CAS No.:113472-19-8
- Rhein-8-glucoside calcium salt
Catalog No.:BCN6349
CAS No.:113443-70-2
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
- 1,2,3,19-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1615
CAS No.:113558-03-5
- Ikarisoside F
Catalog No.:BCN2284
CAS No.:113558-14-8
- Baohuoside I
Catalog No.:BCN5350
CAS No.:113558-15-9
- E-4031 dihydrochloride
Catalog No.:BCC7182
CAS No.:113559-13-0
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- Tigecycline mesylate
Catalog No.:BCC4229
CAS No.:1135871-27-0
- Orbifloxacin
Catalog No.:BCC4689
CAS No.:113617-63-3
Polyester fibers can be rendered calcium phosphate-binding by surface functionalization with bisphosphonate groups.[Pubmed:28371150]
J Biomed Mater Res A. 2017 Aug;105(8):2335-2342.
Fibers are often used as structural elements to improve the mechanical properties of materials such as brittle ceramic matrices by facilitating the dissipation of energy. However, this energy dissipation is mainly controlled by the interface between the two components, and a poorly designed fiber-matrix interface strongly reduces the efficacy of fiber reinforcement. Here, we present a versatile approach to control the affinity of biocompatible fibers to calcium-containing matrices to maximize the efficacy of reinforcement of calcium phosphates-based bioceramics by means of polymeric fibers. To this end, polyester fibers of tunable length were produced by electrospinning and aminolysis, followed by covalent attachment of alendronate, a bisphosphonate molecule with strong calcium-binding affinity, to the surface of the fibers. The proposed method allowed for selective control over the amount of alendronate conjugation, thereby improving the affinity of polyester fibers toward calcium phosphate bioceramics. (c) 2017 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 105A: 2335-2342, 2017.
Fabrication of self-setting beta-tricalcium phosphate granular cement.[Pubmed:28370963]
J Biomed Mater Res B Appl Biomater. 2018 Feb;106(2):800-807.
Bone defect reconstruction would be greatly improved if beta-tricalcium phosphate (beta-TCP) granules had the ability to self-set without sacrificing their osteoconductivity potential. This study aimed to identify a method to permit beta-TCP self-setting whilst maintaining good osteoconductivity. When mixed with acidic calcium phosphate solution, beta-TCP granules were found to readily set, forming a fully interconnected porous structure. On mixing, dicalcium phosphate dihydrate crystals formed on the surface of beta-TCP granules, bridging the granules and resulting in the setting reaction. The setting time of the beta-TCP granular cement (beta-TCP GC) was approximately 1 min and its mechanical strength, in terms of diametral tensile strength, was approximately 0.8 MPa. The beta-TCP GC and beta-TCP granules both showed the same level of osteoconductivity within rat calvaria bone defects. At 2 and 4 weeks post-implantation, new bone formation was comparable between the two beta-TCP based bone substitutes. We conclude that beta-TCP GC has excellent potential for use as a cement in bone defect reconstruction. (c) 2017 Wiley Periodicals, Inc. J Biomed Mater Res Part B: Appl Biomater, 106B: 800-807, 2018.
Highly Time-Resolved Metabolic Reprogramming toward Differential Levels of Phosphate in Chlamydomonas reinhardtii.[Pubmed:28372038]
J Microbiol Biotechnol. 2017 Jun 28;27(6):1150-1156.
Understanding phosphorus metabolism in photosynthetic organisms is important as it is closely associated with enhanced crop productivity and pollution management for natural ecosystems (e.g., algal blooming). Accordingly, we exploited highly time-resolved metabolic responses to different levels of phosphate deprivation in Chlamydomonas reinhardtii, a photosynthetic model organism. We conducted non-targeted primary metabolite profiling using gas-chromatography time-of-flight mass spectrometric analysis. Primarily, we systematically identified main contributors to degree-wise responses corresponding to the levels of phosphate deprivation. Additionally, we systematically characterized the metabolite sets specific to different phosphate conditions and their interactions with culture time. Among them were various types of fatty acids that were most dynamically modulated by the phosphate availability and culture time in addition to phosphorylated compounds.
OsPAP26 Encodes a Major Purple Acid Phosphatase and Regulates Phosphate Remobilization in Rice.[Pubmed:28371895]
Plant Cell Physiol. 2017 May 1;58(5):885-892.
During phosphate (Pi) starvation or leaf senescence, the accumulation of intracellular and extracellular purple acid phosphatases (PAPs) increases in plants in order to scavenge organic phosphorus (P). In this study, we demonstrated that a PAP-encoding gene in rice, OsPAP26, is constitutively expressed in all tissues. While the abundance of OsPAP26 transcript is not affected by Pi supply, it is up-regulated during leaf senescence. Furthermore, Pi deprivation and leaf senescence greatly increased the abundance of OsPAP26 protein. Overexpression or RNA interference (RNAi) of OsPAP26 in transgenic rice significantly increased or reduced APase activities, respectively, in leaves, roots and growth medium. Compared with wild-type (WT) plants, Pi concentrations of OsPAP26-overexpressing plants increased in the non-senescing leaves and decreased in the senescing leaves. The increased remobilization of Pi from the senescing leaves to non-senescing leaves in the OsPAP26-overexpressing plants resulted in better growth performance when plants were grown in Pi-depleted condition. In contrast, OsPAP26-RNAi plants retained more Pi in the senescing leaves, and were more sensitive to Pi starvation stress. OsPAP26 was found to localize to the apoplast of rice cells. Western blot analysis of protein extracts from callus growth medium confirmed that OsPAP26 is a secreted PAP. OsPAP26-overexpressing plants were capable of converting more ATP into inorganic Pi in the growth medium, which further supported the potential role of OsPAP26 in utilizing organic P in the rhizosphere. In summary, we concluded that OsPAP26 performs dual functions in plants: Pi remobilization from senescing to non-senescing leaves; and organic P utilization.
KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N -methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels.[Pubmed:16368898]
J Pharmacol Exp Ther. 2006 Apr;317(1):292-9.
The effect of Ca(2+)/calmodulin-dependent protein kinase II (CaMK II) on voltage-gated ion channels is widely studied through the use of specific CaMK II blockers such as 2-[N-(2-hydroxyethyl)]-N-(4methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-m ethylbenzylamine (KN-93). The present study demonstrates that KN-93 is a direct extracellular blocker of a wide range of cloned Kv channels from a number of different subfamilies. In all channels tested, the effect of 1 microM KN-93 was independent of CaMK II because 1 microM2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylam ine, phosphate (KN-92), an inactive analog of KN-93, caused similar inhibition of currents. In addition, dialysis of cells with 10 microM CaMK II inhibitory peptide fragment 281-301 (CIP) had no effect on current kinetics and did not prevent the inhibitory effect of KN-93. The IC(50) for block of the Kv1.5 channel (used as an example to determine the nature of KN-93 block) was 307 +/- 12 nM. KN-93 blocked open channels with little voltage dependence that did not alter the V(1/2) of channel activation. Removal of P/C-type inactivation by mutation of arginine 487 to valine in the outer pore region of Kv1.5 (R487V) greatly reduced KN-93 block, whereas enhancement of inactivation induced by mutation of threonine 462 to cysteine (T462C) increased the potency of KN-93 by 4-fold. This suggested that KN-93 acted through promotion and stabilization of C-type inactivation. Importantly, KN-93 was ineffective as a blocker when applied intracellularly, suggesting that CaMK II-independent effects of KN-93 on Kv channels can be circumvented by intracellular application of KN-93.


