OrbifloxacinCAS# 113617-63-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113617-63-3 | SDF | Download SDF |
| PubChem ID | 60605 | Appearance | Powder |
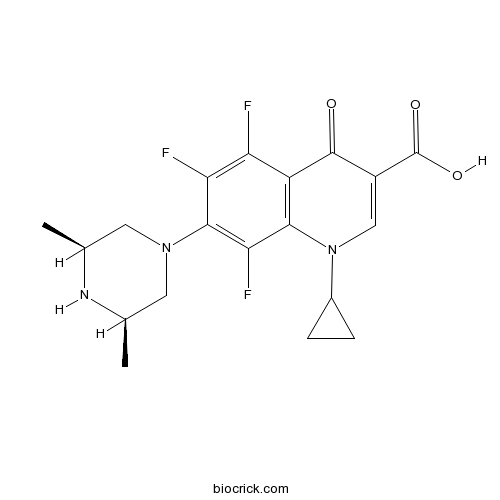
| Formula | C19H20F3N3O3 | M.Wt | 395.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CP-104354 | ||
| Solubility | DMSO : 6 mg/mL (15.18 mM; Need ultrasonic and warming) | ||
| Chemical Name | 1-cyclopropyl-7-[(3S,5R)-3,5-dimethylpiperazin-1-yl]-5,6,8-trifluoro-4-oxoquinoline-3-carboxylic acid | ||
| SMILES | C[C@@H]1CN(C[C@H](C)N1)c2c(F)c(F)c3C(=O)C(=CN(C4CC4)c3c2F)C(O)=O | ||
| Standard InChIKey | QIPQASLPWJVQMH-DTORHVGOSA-N | ||
| Standard InChI | InChI=1S/C19H20F3N3O3/c1-8-5-24(6-9(2)23-8)17-14(21)13(20)12-16(15(17)22)25(10-3-4-10)7-11(18(12)26)19(27)28/h7-10,23H,3-6H2,1-2H3,(H,27,28)/t8-,9+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Orbifloxacin is a synthetic broad-spectrum fluoroquinolone antibiotic which is approved for use in dogs. |

Orbifloxacin Dilution Calculator

Orbifloxacin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5292 mL | 12.6461 mL | 25.2921 mL | 50.5842 mL | 63.2303 mL |
| 5 mM | 0.5058 mL | 2.5292 mL | 5.0584 mL | 10.1168 mL | 12.6461 mL |
| 10 mM | 0.2529 mL | 1.2646 mL | 2.5292 mL | 5.0584 mL | 6.323 mL |
| 50 mM | 0.0506 mL | 0.2529 mL | 0.5058 mL | 1.0117 mL | 1.2646 mL |
| 100 mM | 0.0253 mL | 0.1265 mL | 0.2529 mL | 0.5058 mL | 0.6323 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Orbifloxacin is a synthetic broad-spectrum fluoroquinolone antibiotic.
- Tigecycline mesylate
Catalog No.:BCC4229
CAS No.:1135871-27-0
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- E-4031 dihydrochloride
Catalog No.:BCC7182
CAS No.:113559-13-0
- Baohuoside I
Catalog No.:BCN5350
CAS No.:113558-15-9
- Ikarisoside F
Catalog No.:BCN2284
CAS No.:113558-14-8
- 1,2,3,19-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1615
CAS No.:113558-03-5
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- 6beta-(Hexa-2,4-dienoyloxy)-9alpha,12-dihydroxydrimenol
Catalog No.:BCN7277
CAS No.:1136245-81-2
- Metasequoic acid A
Catalog No.:BCN6652
CAS No.:113626-22-5
- Stigmast-4-ene-3,6-diol
Catalog No.:BCN6014
CAS No.:113626-76-9
- IDE 2
Catalog No.:BCC6099
CAS No.:1136466-93-7
- Ustusolate A
Catalog No.:BCN6756
CAS No.:1136611-58-9
- Neuropeptide Y 13-36 (porcine)
Catalog No.:BCC6959
CAS No.:113662-54-7
- 3-(hydroxymethyl)cyclopentanone
Catalog No.:BCN6015
CAS No.:113681-11-1
- Shizukanolide H
Catalog No.:BCN6016
CAS No.:1136932-34-7
- 4-Aminobenzophenone
Catalog No.:BCC8684
CAS No.:1137-41-3
- BOC-D-ARG-OH.HCL.H2O
Catalog No.:BCC3069
CAS No.:113712-06-4
- Tenatoprazole
Catalog No.:BCC4732
CAS No.:113712-98-4
- cis-Ned 19
Catalog No.:BCC6089
CAS No.:1137264-00-6
Determination of minimum biofilm eradication concentrations of orbifloxacin for canine bacterial uropathogens over different treatment periods.[Pubmed:28042660]
Microbiol Immunol. 2017 Jan;61(1):17-22.
Biofilm formation can cause refractory urinary tract infections (UTIs) in dogs; however, minimum biofilm eradication concentrations (MBECs) of veterinary drugs against canine uropathogens remain to be investigated. In this study, the MBECs of Orbifloxacin (OBFX), trimethoprim-sulfamethoxazole (TMS) and amoxicillin/clavulanate (ACV) over different time periods for treatment of canine uropathogenic Escherichia coli (n = 10) were determined. The MBECs of OBFX for other bacterial uropathogens, including Staphylococcus pseudintermedius (n = 5), Pseudomonas aeruginosa (n = 5), Klebsiella pneumoniae (n = 5) and Proteus mirabilis (n = 5) were also determined. Minimum inhibitory concentrations (MICs) were identified for all strains by broth microdilution, and MBECs were determined at 24, 72, and 168 hr using the Calgary biofilm method. The 24 hr MBECs of OBFX, TMS and ACV for the E. coli strains were significantly higher than the MICs (P < 0.05), and the 72 and 168 hr MBECs were significantly lower than those at 24 hr (P < 0.05). In addition, the 24 hr OBFX MBECs for the four other uropathogens were significantly higher than the corresponding MICs (P < 0.05). The 72 and/or 168 hr OBFX MBECs for S. pseudintermedius, K. pneumoniae and P. mirabilis were significantly lower than the 24 hr concentrations (P < 0.05), whereas for P. aeruginosa, no significant difference was found between any of the MBECs (P > 0.05). These data indicate that the administration period and uropathogenic bacterial species are important factors affecting the efficacy of OBFX treatment of biofilm-related UTIs in dogs.
Pharmacokinetics and bioavailability of orbifloxacin oral suspension in New Zealand White rabbits (Oryctolagus cuniculus).[Pubmed:26512539]
Am J Vet Res. 2015 Nov;76(11):946-51.
OBJECTIVE To evaluate the pharmacokinetics and bioavailability of 2 doses of Orbifloxacin in rabbits. ANIMALS 6 healthy purpose-bred adult female New Zealand White rabbits (Oryctolagus cuniculus). PROCEDURES Each of 3 rabbits received Orbifloxacin at either 10 or 20 mg/kg, PO. Then, after a 1-week washout period, they received the same dose IV. Blood samples were collected from each rabbit at 0, 0.25, 0.5, 1, 2, 4, 6, 12, and 24 hours after drug administration. Plasma Orbifloxacin concentration was measured with liquid chromatography-tandem mass spectrometry. Pharmacokinetic parameters were determined by noncompartmental analysis for data obtained following PO administration and noncompartmental and compartmental analyses for data obtained following IV administration. RESULTS Following oral administration, the mean +/- SD peak plasma Orbifloxacin concentration was 1.66 +/- 0.51 mug/mL for rabbits administered the 10 mg/kg dose and 3.00 +/- 0.97 mug/mL for rabbits administered the 20 mg/kg dose and was attained at 2 hours after drug administration. The mean +/- SD half-life of Orbifloxacin in plasma was 7.3 +/- 1.1 hours for rabbits administered the 10 mg/kg dose and 8.6 +/- 0.55 hours for rabbits administered the 20 mg/kg dose. Mean bioavailability was 52.5% for rabbits administered the 10 mg/kg dose and 46.5% for rabbits administered the 20 mg/kg dose. CONCLUSIONS AND CLINICAL RELEVANCE Results provided pharmacokinetic properties for 2 doses (10 mg/kg and 20 mg/kg) of Orbifloxacin oral suspension in rabbits. Further studies are necessary to determine the protein-binding activity of Orbifloxacin in rabbits before dosages for the treatment of common pathogens in this species are recommended.
Effects of oral orbifloxacin on fecal coliforms in healthy cats: a pilot study.[Pubmed:26311787]
J Vet Med Sci. 2016 Jan;78(1):83-9.
The study objective was to determine the effect of oral Orbifloxacin (ORB) on antimicrobial susceptibility and composition of fecal coliforms in cats. Nine cats were randomized to two groups administered a daily oral dose of 2.5 and 5.0 mg ORB/kg for 7 days and a control group (three cats per group). Coliforms were isolated from stool samples and were tested for susceptibilities to ORB and 5 other drugs. ORB concentration in feces was measured using high-performance liquid chromatography (HPLC). The coliforms were undetectable after 2 days of ORB administration, and their number increased in most cats after termination of the administration. Furthermore, only isolates of Escherichia coli were detected in all cats before administration, and those of Citrobacter freundii were detected after termination of the administration. E. coli isolates exhibited high ORB susceptibility [Minimum inhibitory concentration (MIC),
Structure, Solubility and Stability of Orbifloxacin Crystal Forms: Hemihydrate versus Anhydrate.[Pubmed:27005603]
Molecules. 2016 Mar 9;21(3):328.
Orbifloxacin (ORBI) is a widely used antimicrobial drug of the fluoroquinolone class. In the official pharmaceutical compendia the existence of polymorphism in this active pharmaceutical ingredient (API) is reported. No crystal structure has been reported for this API and as described in the literature, its solubility is very controversial. Considering that different solid forms of the same API may have different physicochemical properties, these different solubilities may have resulted from analyses inadvertently carried out on different polymorphs. The solubility is the most critical property because it can affect the bioavailability and may compromise the quality of a drug product. The crystalline structure of ORBI determined by SCXRD is reported here for the first time. The structural analysis reveals that the ORBI molecule is zwitterionic and hemihydrated. ORBI hemihydrated form was characterized by the following techniques: TG/DTA, FTIR-ATR, and PXRD. A second crystalline ORBI form is also reported: the ORBI anhydrous form was obtained by heating the hemihydrate. These ORBI solid forms were isomorphous, since no significant change in unit cell and space group symmetry were observed. The solid-state phase transformation between these forms is discussed and the equilibrium solubility data were examined in order to check the impact of the differences observed in their crystalline structures.


