IDE 2Induces definitive endoderm formation in mouse and human ESCs CAS# 1136466-93-7 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1136466-93-7 | SDF | Download SDF |
| PubChem ID | 23836158 | Appearance | Powder |
| Formula | C12H20N2O3 | M.Wt | 240.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in water and to 100 mM in DMSO | ||
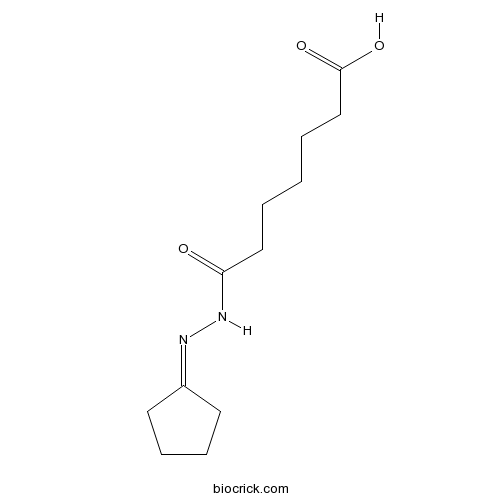
| Chemical Name | 7-(2-cyclopentylidenehydrazinyl)-7-oxoheptanoic acid | ||
| SMILES | C1CCC(=NNC(=O)CCCCCC(=O)O)C1 | ||
| Standard InChIKey | CVYPYYPTFNVREL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H20N2O3/c15-11(8-2-1-3-9-12(16)17)14-13-10-6-4-5-7-10/h1-9H2,(H,14,15)(H,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cell-permeable inducer of definitive endoderm formation in mouse and human embryonic stem cells (ESCs) (EC50 = 223 nM for induction of Sox17 expression in ESCs). Reported to activate TGF-β signaling and downstream Smad2 phosphorylation; upregulates Nodal expression. Promotes pancreatic progenitor cell formation in vitro, and gut tube formation in vivo. |

IDE 2 Dilution Calculator

IDE 2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1615 mL | 20.8073 mL | 41.6146 mL | 83.2293 mL | 104.0366 mL |
| 5 mM | 0.8323 mL | 4.1615 mL | 8.3229 mL | 16.6459 mL | 20.8073 mL |
| 10 mM | 0.4161 mL | 2.0807 mL | 4.1615 mL | 8.3229 mL | 10.4037 mL |
| 50 mM | 0.0832 mL | 0.4161 mL | 0.8323 mL | 1.6646 mL | 2.0807 mL |
| 100 mM | 0.0416 mL | 0.2081 mL | 0.4161 mL | 0.8323 mL | 1.0404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stigmast-4-ene-3,6-diol
Catalog No.:BCN6014
CAS No.:113626-76-9
- Metasequoic acid A
Catalog No.:BCN6652
CAS No.:113626-22-5
- 6beta-(Hexa-2,4-dienoyloxy)-9alpha,12-dihydroxydrimenol
Catalog No.:BCN7277
CAS No.:1136245-81-2
- Orbifloxacin
Catalog No.:BCC4689
CAS No.:113617-63-3
- Tigecycline mesylate
Catalog No.:BCC4229
CAS No.:1135871-27-0
- Q-VD-OPh hydrate
Catalog No.:BCC1125
CAS No.:1135695-98-5
- E-4031 dihydrochloride
Catalog No.:BCC7182
CAS No.:113559-13-0
- Baohuoside I
Catalog No.:BCN5350
CAS No.:113558-15-9
- Ikarisoside F
Catalog No.:BCN2284
CAS No.:113558-14-8
- 1,2,3,19-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1615
CAS No.:113558-03-5
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- Ustusolate A
Catalog No.:BCN6756
CAS No.:1136611-58-9
- Neuropeptide Y 13-36 (porcine)
Catalog No.:BCC6959
CAS No.:113662-54-7
- 3-(hydroxymethyl)cyclopentanone
Catalog No.:BCN6015
CAS No.:113681-11-1
- Shizukanolide H
Catalog No.:BCN6016
CAS No.:1136932-34-7
- 4-Aminobenzophenone
Catalog No.:BCC8684
CAS No.:1137-41-3
- BOC-D-ARG-OH.HCL.H2O
Catalog No.:BCC3069
CAS No.:113712-06-4
- Tenatoprazole
Catalog No.:BCC4732
CAS No.:113712-98-4
- cis-Ned 19
Catalog No.:BCC6089
CAS No.:1137264-00-6
- BRD 7552
Catalog No.:BCC8035
CAS No.:1137359-47-7
- LX1606
Catalog No.:BCC1713
CAS No.:1137608-69-5
- Ilexhainanoside D
Catalog No.:BCN7863
CAS No.:1137648-52-2
- Eudesm-4(15)-ene-3alpha,11-diol
Catalog No.:BCN4060
CAS No.:113773-90-3
2-(Pyridinium-1-yl)-1,1-bis(perfluoroalkylsulfonyl)ethan-1-ide: A Practical Reagent for Synthesis of Strongly Acidic 1,1-Bis(perfluoroalkylsulfonyl)alkanes.[Pubmed:28266793]
Chemistry. 2017 Jun 16;23(34):8203-8211.
On mixing (Rf SO2 )2 CH2 (Rf =perfluoroalkyl), paraformaldehyde, and substituted pyridines, a three-component reaction proceeded smoothly to give unusual zwitterions bearing both pyridinium and stabilized carbanion moieties in good to excellent yields. Of these, 2-fluoropyridinium derivatives rapidly dissociated in acetonitrile to give equilibrium mixtures of the zwitterions and (Rf SO2 )2 C=CH2 /2-fluoropyridine, as confirmed by detailed variable-temperature NMR studies. The dynamic behavior of such 2-fluoropyridinium compounds allows them to be used as shelf-stable, easy-to-handle sources of (Rf SO2 )2 C=CH2 . With these reagents, strongly acidic carbon acids (Rf SO2 )2 CHR were synthesized, which served as a new type of acid catalysts. Moreover, C-C bond-forming reactions with a ketene silyl acetal proceeded efficiently with Tf2 C=CH2 generated in situ.
First Synthesis of Novel Aminophenyl Pyridinium-5-(hydroxybenzoyl)-hydrazonomethyl-2-oxothiazol-3-ide Derivatives and Evaluation of Their Anticancer Activities.[Pubmed:26235251]
Chem Pharm Bull (Tokyo). 2015;63(10):843-7.
The first total synthesis for large-scale production and anticancer activity of novel aminophenylpyridinium-5-(hydroxybenzoyl)hydrazonomethyl-2-oxothiazol-3-ide (PBHT) (1) and its derivatives are reported. The chemical structure of PBHT was unambiguously determined by utilization of the two-dimensional nuclear Overhauser effect (NOE) technique. The anticancer activity against human colon adenocarcinoma (HCT15) cells of all synthesized compounds was approximately four-fold greater than that of 5-fluorouracil, with IC50 values ranging from 10.1 to 14.2 microM. The three structural determinants of hydroxybenzoyl, hydrazinylidene, and pyridinium oxothiazole in the synthesized compounds could be indispensable for exhibiting anticancer activity.
An efficient synthetic method for organometallic radicals: structures and properties of gold(i)-(nitronyl nitroxide)-2-ide complexes.[Pubmed:28165514]
Dalton Trans. 2017 Feb 21;46(8):2653-2659.
One-pot synthesis of (nitronyl nitroxide)-gold(i)-phosphine (NN-Au-P) complexes has been developed using chloro(tetrahydrothiophene)gold(i), phosphine ligands, nitronyl nitroxide radicals, and sodium hydroxide. The NN-Au-P complexes can be easily handled because they were quite stable under aerated conditions in both solution and crystalline states. They showed weak absorption bands with vibrational structures in the 450-650 nm region. The oxidation potentials assigned to the NN moieties of NN-Au-P complexes with aromatic phosphines were observed around -0.1 V vs. Fc/Fc(+) (-0.11 V for NN-Au-1, -0.08 V for NN-Au-2, -0.13 V for NN-Au-5, and -0.07 V for NN-Au-6), somewhat lower than that of NN-Au-P complexes with aliphatic phosphines (-0.25 V for NN-Au-3 and -0.17 V for NN-Au-4).
Crystal structures of 4-phenyl-piperazin-1-ium 6-chloro-5-ethyl-2,4-dioxopyrimidin-1-ide and 4-phenyl-piperazin-1-ium 6-chloro-5-isopropyl-2,4-dioxopyrimidin-1-ide.[Pubmed:26396765]
Acta Crystallogr E Crystallogr Commun. 2015 Jul 22;71(Pt 8):956-9.
The title mol-ecular salts, C10H15N2 (+).C6H6ClN2O2 (-), (I), and C10H15N2 (+).C7H8ClN2O2 (-), (II), consist of 4-phenyl-piperazin-1-ium cations with a 6-chloro-5-ethyl-2,4-dioxopyrimidin-1-ide anion in (I) and a 6-chloro-5-isopropyl-2,4-dioxopyrimidin-1-ide anion in (II). Salt (I) crystallizes with two independent cations and anions in the asymmetric unit. In the crystal structures of both salts, the ions are linked via N-Hcdots, three dots, centeredO and N-Hcdots, three dots, centeredN hydrogen bonds, forming sheets which are parallel to (100) in (I) and to (001) in (II). In (I), the sheets are linked via C-Hcdots, three dots, centeredCl hydrogen bonds, forming a three-dimensional framework.
Stimulation of Activin A/Nodal signaling is insufficient to induce definitive endoderm formation of cord blood-derived unrestricted somatic stem cells.[Pubmed:21463501]
Stem Cell Res Ther. 2011 Apr 4;2(2):16.
INTRODUCTION: Unrestricted somatic stem cells (USSC) derived from umbilical cord blood are an attractive alternative to human embryonic stem cells (hESC) for cellular therapy. USSC are capable of forming cells representative of all three germ line layers. The aim of this study was to determine the potential of USSC to form definitive endoderm following induction with Activin A, a protein known to specify definitive endoderm formation of hESC. METHODS: USSC were cultured for (1) three days with or without 100 ng/ml Activin A in either serum-free, low-serum or serum-containing media, (2) three days with or without 100 ng/ml Activin A in combination with 10 ng/ml FGF4 in pre-induction medium, or (3) four days with or without small molecules Induce Definitive Endoderm (IDE1, 100 nM; IDE2, 200 nM) in serum-free media. Formation of definitive endoderm was assessed using RT-PCR for gene markers of endoderm (Sox17, FOXA2 and TTF1) and lung epithelium (surfactant protein C; SPC) and cystic fibrosis transmembrane conductance regulator; CFTR). The differentiation capacity of Activin A treated USSC was also assessed. RESULTS: Activin A or IDE1/2 induced formation of Sox17+ definitive endoderm from hESC but not from USSC. Activin A treated USSC retained their capacity to form cells of the ectoderm (nerve), mesoderm (bone) and endoderm (lung). Activin A in combination with FGF4 did not induce formation of Sox17+ definitive endoderm from USSC. USSC express both Activin A receptor subunits at the mRNA and protein level, indicating that these cells are capable of binding Activin A. CONCLUSIONS: Stimulation of the Nodal signaling pathway with Activin A or IDE1/2 is insufficient to induce definitive endoderm formation from USSC, indicating that USSC differ in their stem cell potential from hESC.
Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells.[Pubmed:19341624]
Cell Stem Cell. 2009 Apr 3;4(4):348-58.
An essential step for therapeutic and research applications of stem cells is the ability to differentiate them into specific cell types. Endodermal cell derivatives, including lung, liver, and pancreas, are of interest for regenerative medicine, but efforts to produce these cells have been met with only modest success. In a screen of 4000 compounds, two cell-permeable small molecules were indentified that direct differentiation of ESCs into the endodermal lineage. These compounds induce nearly 80% of ESCs to form definitive endoderm, a higher efficiency than that achieved by Activin A or Nodal, commonly used protein inducers of endoderm. The chemically induced endoderm expresses multiple endodermal markers, can participate in normal development when injected into developing embryos, and can form pancreatic progenitors. The application of small molecules to differentiate mouse and human ESCs into endoderm represents a step toward achieving a reproducible and efficient production of desired ESC derivatives.
Using small molecules to great effect in stem cell differentiation.[Pubmed:19427285]
Cell Stem Cell. 2009 May 8;4(5):373-4.
Several recent reports, including two Cell Stem Cell papers (Zhu et al., 2009 [this issue]; Borowiak et al., 2009), screened small molecule libraries for compounds that promote embryonic stem cell differentiation. Their combined success helps bypass challenges associated with using natural protein factors and has revealed insights into controlling stem cell differentiation.


