Dexrazoxane HCl (ICRF-187, ADR-529)Topoisomerase II inhibitor,intracellular ion chelator,cardioprotective agent CAS# 149003-01-0 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 149003-01-0 | SDF | Download SDF |
| PubChem ID | 6918223 | Appearance | Powder |
| Formula | C11H17ClN4O4 | M.Wt | 304.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Dexrazoxane hydrochloride; Cardioxane; Dexrazoxane HCl; Zinecard; ICRF-187 hydrochloride;dexrazoxane | ||
| Solubility | DMSO : 50 mg/mL (Need ultrasonic) H2O : 20 mg/mL (Need ultrasonic) | ||
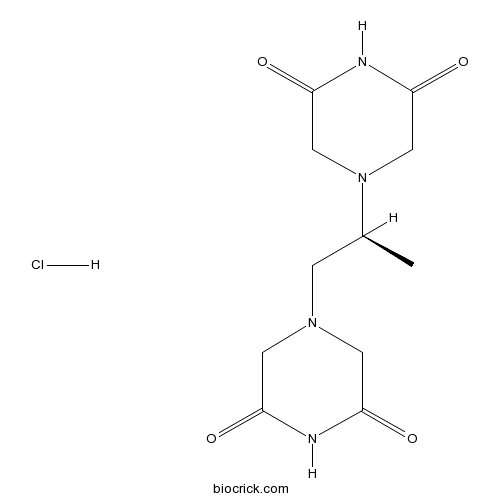
| Chemical Name | 4-[(2S)-2-(3,5-dioxopiperazin-1-yl)propyl]piperazine-2,6-dione;hydrochloride | ||
| SMILES | CC(CN1CC(=O)NC(=O)C1)N2CC(=O)NC(=O)C2.Cl | ||
| Standard InChIKey | BIFMNMPSIYHKDN-FJXQXJEOSA-N | ||
| Standard InChI | InChI=1S/C11H16N4O4.ClH/c1-7(15-5-10(18)13-11(19)6-15)2-14-3-8(16)12-9(17)4-14;/h7H,2-6H2,1H3,(H,12,16,17)(H,13,18,19);1H/t7-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dexrazoxane Hcl( ICRF-187 Hcl) is a cardioprotective agent. IC50 value: Target: cardioprotective agent As a derivative of EDTA, dexrazoxane chelates iron, thus reduce the number of metal ions complexed with anthracycline and, consequently, decrease the formation of superoxide radicals. This agent is used to protect the heart against the cardiotoxic side effects of anthracyclines, such as doxorubicin. It was speculated that dexrazoxane could be used for further investigation to synthesize new antimalarial drugs. | |||||

Dexrazoxane HCl (ICRF-187, ADR-529) Dilution Calculator

Dexrazoxane HCl (ICRF-187, ADR-529) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2816 mL | 16.408 mL | 32.8159 mL | 65.6319 mL | 82.0398 mL |
| 5 mM | 0.6563 mL | 3.2816 mL | 6.5632 mL | 13.1264 mL | 16.408 mL |
| 10 mM | 0.3282 mL | 1.6408 mL | 3.2816 mL | 6.5632 mL | 8.204 mL |
| 50 mM | 0.0656 mL | 0.3282 mL | 0.6563 mL | 1.3126 mL | 1.6408 mL |
| 100 mM | 0.0328 mL | 0.1641 mL | 0.3282 mL | 0.6563 mL | 0.8204 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dexrazoxane Hydrochloride is the hydrochloride salt of a bisdioxopiperazine with iron-chelating, chemoprotective, cardioprotective, and antineoplastic activities. After hydrolysis to an active form that is similar to ethylenediaminetetraacetic acid (EDTA), dexrazoxane chelates iron, limiting the formation of free radical-generating anthracycline-iron complexes, which may minimize anthracycline-iron complex-mediated oxidative damage to cardiac and soft tissues. This agent also inhibits the catalytic activity of topoisomerase II, which may result in tumor cell growth inhibition.
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- Gallic acid
Catalog No.:BCN1668
CAS No.:149-91-7
- Scopolamine butylbromide
Catalog No.:BCN5006
CAS No.:149-64-4
- Erythritol
Catalog No.:BCN1664
CAS No.:149-32-6
- Fmoc-Inp-OH
Catalog No.:BCC3266
CAS No.:148928-15-8
- 3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl-2-propenal
Catalog No.:BCC8600
CAS No.:148901-68-2
- Fmoc-L-Arg(Aloc)2-OH
Catalog No.:BCC2564
CAS No.:148893-34-9
- HATU
Catalog No.:BCC2813
CAS No.:148893-10-1
- YM 511
Catalog No.:BCC6002
CAS No.:148869-05-0
- Ivabradine HCl
Catalog No.:BCC4350
CAS No.:148849-67-6
- Rutamarin
Catalog No.:BCN7509
CAS No.:14882-94-1
- Bismuth Subsalicylate
Catalog No.:BCC3739
CAS No.:14882-18-9
- (S)-WAY 100135 dihydrochloride
Catalog No.:BCC6993
CAS No.:149007-54-5
- H-D-Trp-OMe.HCl
Catalog No.:BCC3118
CAS No.:14907-27-8
- Brusatol
Catalog No.:BCN8278
CAS No.:14907-98-3
- AG 825
Catalog No.:BCC7113
CAS No.:149092-50-2
- Brugine
Catalog No.:BCN1899
CAS No.:14912-30-2
- Cratoxylone
Catalog No.:BCN3875
CAS No.:149155-01-1
- Homaloside D
Catalog No.:BCN1661
CAS No.:149155-19-1
- Eriodictyol chalcone
Catalog No.:BCN8276
CAS No.:14917-41-0
- 3-(2,4-Dihydroxybenzyl)-5-hydroxy-7,8-dimethoxy-6-methylchroman-4-one
Catalog No.:BCN6634
CAS No.:149180-48-3
- Irenolone
Catalog No.:BCN7146
CAS No.:149184-19-0
- CGP 54626 hydrochloride
Catalog No.:BCC6934
CAS No.:149184-21-4
- CGP 55845 hydrochloride
Catalog No.:BCC5737
CAS No.:149184-22-5
The use of dexrazoxane for the prevention of anthracycline extravasation injury.[Pubmed:18230055]
Expert Opin Investig Drugs. 2008 Feb;17(2):217-23.
BACKGROUND: The use of the anthracycline anticancer drugs doxorubicin, daunorubicin, epirubicin and idarubicin sometimes results in accidental extravasation injury and can be a serious complication of their use. OBJECTIVE: The object of this review was to evaluate the preclinical and clinical literature on the use of dexrazoxane in preventing anthracycline-induced extravasation injury. METHODS: A review of the literature was carried out using PubMed. RESULTS/CONCLUSIONS: Dexrazoxane, which is clinically used to reduce doxorubicin-induced cardiotoxicity, has been shown in two clinical studies and in several case reports to be highly efficacious in preventing anthracycline-induced extravasation injury. Dexrazoxane is a prodrug analog of the metal chelator EDTA that likely acts by removing iron from the iron-anthracycline complex, thus preventing formation of damaging reactive oxygen species.
Utility of dexrazoxane for the reduction of anthracycline-induced cardiotoxicity.[Pubmed:19018683]
Expert Rev Cardiovasc Ther. 2008 Nov;6(10):1311-7.
Dexrazoxane is a derivative of the powerful metal-chelating agent ethyl enediamine tetra acetic acid. Its cardioprotective effect is thought to be due to its ability to chelate iron and reduce the number of metal ions complexed with anthracycline and, consequently, decrease the formation of superoxide radicals. Preclinical studies have confirmed that dexrazoxane has significant activity as a cardioprotective agent against anthracycline-induced cardiotoxicity. Dexrazoxane is well-tolerated, with myelosuppression being the dose-limiting toxicity in Phase I trials. The cardioprotective utility of dexrazoxane has been further illustrated in a number of randomized trials. In addition no significant difference in survival has been observed between the dexrazoxane and control arms of these trials but, in one, a significantly lower response rate was observed in the dexrazoxane compared to placebo arm. Further trials are required to evaluate the efficacy of dexrazoxane in hematological malignancies as well as the adjuvant treatment of breast cancer. Its use in the paediatric setting and in the management of elderly patients with cardiac comorbidity also requires investigation. Recently, interest has focused on the use of dexrazoxane as an antidote for anthracycline extravasation. In addition the general cytoprotective activity of this drug requires further assessment, as well as selectivity in this context.
Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial.[Pubmed:20850381]
Lancet Oncol. 2010 Oct;11(10):950-61.
BACKGROUND: Doxorubicin chemotherapy is associated with cardiomyopathy. Dexrazoxane reduces cardiac damage during treatment with doxorubicin in children with acute lymphoblastic leukaemia (ALL). We aimed to establish the long-term effect of dexrazoxane on the subclinical state of cardiac health in survivors of childhood high-risk ALL 5 years after completion of doxorubicin treatment. METHODS: Between January, 1996, and September, 2000, children with high-risk ALL were enrolled from nine centres in the USA, Canada, and Puerto Rico. Patients were assigned by block randomisation to receive ten doses of 30 mg/m(2) doxorubicin alone or the same dose of doxorubicin preceded by 300 mg/m(2) dexrazoxane. Treatment assignment was obtained through a telephone call to a centralised registrar to conceal allocation. Investigators were masked to treatment assignment but treating physicians and patients were not; however, investigators, physicians, and patients were masked to study serum cardiac troponin-T concentrations and echocardiographic measurements. The primary endpoints were late left ventricular structure and function abnormalities as assessed by echocardiography; analyses were done including all patients with data available after treatment completion. This trial has been completed and is registered with ClinicalTrials.gov, number NCT00165087. FINDINGS: 100 children were assigned to doxorubicin (66 analysed) and 105 to doxorubicin plus dexrazoxane (68 analysed). 5 years after the completion of doxorubicin chemotherapy, mean left ventricular fractional shortening and end-systolic dimension Z scores were significantly worse than normal for children who received doxorubicin alone (left ventricular fractional shortening: -0.82, 95% CI -1.31 to -0.33; end-systolic dimension: 0.57, 0.21-0.93) but not for those who also received dexrazoxane (-0.41, -0.88 to 0.06; 0.15, -0.20 to 0.51). The protective effect of dexrazoxane, relative to doxorubicin alone, on left ventricular wall thickness (difference between groups: 0.47, 0.46-0.48) and thickness-to-dimension ratio (0.66, 0.64-0.68) were the only statistically significant characteristics at 5 years. Subgroup analysis showed dexrazoxane protection (p=0.04) for left ventricular fractional shortening at 5 years in girls (1.17, 0.24-2.11), but not in boys (-0.10, -0.87 to 0.68). Similarly, subgroup analysis showed dexrazoxane protection (p=0.046) for the left ventricular thickness-to-dimension ratio at 5 years in girls (1.15, 0.44-1.85), but not in boys (0.19, -0.42 to 0.81). With a median follow-up for recurrence and death of 8.7 years (range 1.3-12.1), event-free survival was 77% (95% CI 67-84) for children in the doxorubicin-alone group, and 76% (67-84) for children in the doxorubicin plus dexrazoxane group (p=0.99). INTERPRETATION: Dexrazoxane provides long-term cardioprotection without compromising oncological efficacy in doxorubicin-treated children with high-risk ALL. Dexrazoxane exerts greater long-term cardioprotective effects in girls than in boys. FUNDING: US National Institutes of Health, Children's Cardiomyopathy Foundation, University of Miami Women's Cancer Association, Lance Armstrong Foundation, Roche Diagnostics, Pfizer, and Novartis.


