Bismuth SubsalicylateCAS# 14882-18-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14882-18-9 | SDF | Download SDF |
| PubChem ID | 16682734 | Appearance | Powder |
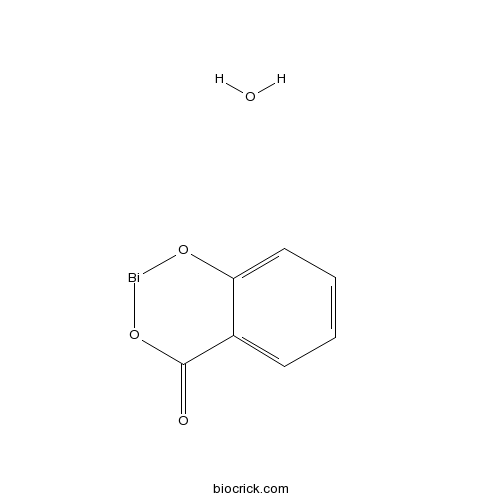
| Formula | C7H5BiO4 | M.Wt | 362.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Bismuth oxysalicylate; Bismuth(III) salicylate basic | ||
| Solubility | DMSO : < 1 mg/mL (insoluble or slightly soluble) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 1,3,2$l^{2}-benzodioxabismin-4-one;hydrate | ||
| SMILES | C1=CC=C2C(=C1)C(=O)O[Bi]O2.O | ||
| Standard InChIKey | QBWLKDFBINPHFT-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C7H6O3.Bi.H2O/c8-6-4-2-1-3-5(6)7(9)10;;/h1-4,8H,(H,9,10);;1H2/q;+2;/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bismuth Subsalicylate is the active ingredient in Pepto-Bismol and inhibits prostaglandin G/H Synthase 1/2.
Target: Others
Bismuth Subsalicylate reduces inflammation/irritation of stomach and intestinal lining through inhibition of prostaglandin G/H Synthase 1/2 [1]. Bismuth Subsalicylate is the active ingredient in Pepto-Bismol, an anti-diarrhea medication and antacid. In the gastrointestinal tract, Bismuth Subsalicylate is converted to salicylic acid and insoluble bismuth salts [2]. Bismuth subsalicylate treatment for 8 weeks is safe and well tolerated. This regimen appears to be efficacious for the treatment of microscopic colitis and is worthy of further study in a controlled trial [3]. References: | |||||

Bismuth Subsalicylate Dilution Calculator

Bismuth Subsalicylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7617 mL | 13.8087 mL | 27.6174 mL | 55.2349 mL | 69.0436 mL |
| 5 mM | 0.5523 mL | 2.7617 mL | 5.5235 mL | 11.047 mL | 13.8087 mL |
| 10 mM | 0.2762 mL | 1.3809 mL | 2.7617 mL | 5.5235 mL | 6.9044 mL |
| 50 mM | 0.0552 mL | 0.2762 mL | 0.5523 mL | 1.1047 mL | 1.3809 mL |
| 100 mM | 0.0276 mL | 0.1381 mL | 0.2762 mL | 0.5523 mL | 0.6904 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bismuth subsalicylate is used to treat diarrhea in adults and teenagers. It is also used to relieve the symptoms of an upset stomach, such as heartburn, indigestion, and nausea in adults and teenagers.
- Carboxy-PTIO, potassium salt
Catalog No.:BCC6789
CAS No.:148819-94-7
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- Tyrphostin AG 879
Catalog No.:BCC4514
CAS No.:148741-30-4
- SB 204070
Catalog No.:BCC5752
CAS No.:148688-01-1
- L-733,060 hydrochloride
Catalog No.:BCC5707
CAS No.:148687-76-7
- GR 127935 hydrochloride
Catalog No.:BCC7081
CAS No.:148642-42-6
- Fmoc-Prolinol
Catalog No.:BCC2710
CAS No.:148625-77-8
- 3-O-Methylquercetin
Catalog No.:BCN1660
CAS No.:1486-70-0
- 3-O-Methylquercetin tetraacetate
Catalog No.:BCN1659
CAS No.:1486-69-7
- 3,5-Dihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1658
CAS No.:14858-07-2
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- GRK2i
Catalog No.:BCC6048
CAS No.:148505-03-7
- Rutamarin
Catalog No.:BCN7509
CAS No.:14882-94-1
- Ivabradine HCl
Catalog No.:BCC4350
CAS No.:148849-67-6
- YM 511
Catalog No.:BCC6002
CAS No.:148869-05-0
- HATU
Catalog No.:BCC2813
CAS No.:148893-10-1
- Fmoc-L-Arg(Aloc)2-OH
Catalog No.:BCC2564
CAS No.:148893-34-9
- 3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl-2-propenal
Catalog No.:BCC8600
CAS No.:148901-68-2
- Fmoc-Inp-OH
Catalog No.:BCC3266
CAS No.:148928-15-8
- Erythritol
Catalog No.:BCN1664
CAS No.:149-32-6
- Scopolamine butylbromide
Catalog No.:BCN5006
CAS No.:149-64-4
- Gallic acid
Catalog No.:BCN1668
CAS No.:149-91-7
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
Saccharomyces boulardii and bismuth subsalicylate as low-cost interventions to reduce the duration and severity of cholera.[Pubmed:26260354]
Pathog Glob Health. 2015 Sep;109(6):275-82.
We conducted a randomised single-blinded clinical trial of 100 cholera patients in Port-au-Prince, Haiti to determine if the probiotic Saccharomyces cerevisiae var. boulardii and the anti-diarrhoeal drug Bismuth Subsalicylate (BS) were able to reduce the duration and severity of cholera. Subjects received either: S. boulardii 250 mg, S. boulardii 250 mg capsule plus BS 524 mg tablet, BS 524 mg, or two placebo capsules every 6 hours alongside standard treatment for cholera. The length of hospitalisation plus the number and volume of emesis, stool and urine were recorded every 6 hours until the study subject was discharged (n = 83), left against medical advice (n = 11), or requested removal from the study (n = 6). There were no reported deaths or adverse study-related events. There were no statistically significant differences between the study arms and the outcomes of interest.
Mitigation of in vitro hydrogen sulfide production using bismuth subsalicylate with and without monensin in beef feedlot diets.[Pubmed:26641054]
J Anim Sci. 2015 Nov;93(11):5346-54.
The objective of this study was to determine if a sulfur binder, Bismuth Subsalicylate (BSS), alone or combined with monensin (MON) could decrease the production of HS by rumen microbes. In Exp. 1, two 24-h batch culture incubations were conducted using a substrate consisting of 50% corn, 40% distillers grains, 9.75% hay, and 0.25% mineral premix, on a DM basis. Five treatments including BSS concentrations of 0% (control), 0.5%, 1%, 2%, and 4% of DM were assigned in 5 replicates to 120-mL serum bottles containing rumen fluid, buffer, and 0.5 g of dietary substrate. Addition of 2% and 4% BSS decreased ( < 0.05) gas production, whereas all concentrations of BSS reduced ( < 0.05) HS production by 18%, 24%, 82%, and 99% for 0.5%, 1%, 2%, and 4% BSS, respectively. Final pH increased ( < 0.05) with 2% and 4% BSS treatments. At 4% of DM, BSS decreased ( < 0.05) total VFA concentration (m) and propionate (mol/100 mol) but increased acetate (mol/100 mol) and acetate to propionate ratio. Concentration of branched-chain VFA increased ( < 0.05) with the addition of 0.5% BSS, compared with the control. On the basis of these results, addition of BSS (1% of DM) and MON (5 mg/kg) were used to assess their effects on metabolism and HS release by rumen microbes in 8 dual flow continuous culture fermenters during two 10-d periods (Exp. 2). Treatments were arranged in a 2 x 2 factorial design. Substrate similar to that used in Exp. 1 was provided at 75 g DM/fermenter daily. Headspace HS concentration was reduced ( < 0.05) by 99% with BSS treatment but was not affected ( = 0.21) by MON. An overall increase ( < 0.05) in fermentation pH was found following addition of BSS. Addition of BSS increased ( < 0.05) digestion of NDF and ADF but decreased ( < 0.05) nonfiber carbohydrate digestion and total VFA concentration. Acetate and propionate (mol/100 mol) increased ( < 0.05) with BSS, whereas butyrate (mol/100 mol) and branched-chain VFA (m) decreased ( < 0.05). Addition of BSS increased ( < 0.05) NH-N concentration and NH-N outflow but decreased ( < 0.05) microbial N outflow. Results from this study showed no response to monensin addition, but BSS markedly reduced HS production and altered microbial fermentation during in vitro rumen fluid incubations.
[Addition of bismuth subsalicylate to triple eradication therapy for Helicobacter pylori infection: efficiency and adverse events].[Pubmed:25594755]
Rev Gastroenterol Peru. 2014 Oct-Dec;34(4):315-20.
OBJECTIVES: To establish the efficiency and adverse effects of the addition of Bismuth Subsalicylate to triple eradication therapy for Helicobacter pylori infection. MATERIAL AND METHODS: Double blind controlled experimental trial. The study population consisted of 54 patients with Helicobacter pylori infection: 29 were allocated to the experimental group, who received the usual triple plus Bismuth Subsalicylate therapy, and 24 received the triple therapy plus placebo. RESULTS: The average age was 47+-14.9 years, 66.7% of the patients were women. Both groups underwent the breath test: it was negative in 89.7% of the patients from the experimental group and 80% of the patients from the placebo group (p=0.319). The adverse events of both groups were: diarrhea (10.3% in the experimental group vs 16% in the placebo group; p=0.537), dark feces (37.9% in the experimental group vs 0% in the placebo group; p=0.001), abdominal pain (20.7% in the experimental group vs 52% in the placebo group; p=0.016). Nausea only were present in 3% of the patients of placebo group p=0.055). CONCLUSIONS: The association of Bismuth Subsalicylate to the triple therapy scheme for the eradication of Helicobacter pylori was effective in 89.7% of patients, whereas 80% of efficiency in the experimental group.
Antimicrobial activity of bismuth subsalicylate on Clostridium difficile, Escherichia coli O157:H7, norovirus, and other common enteric pathogens.[Pubmed:25901890]
Gut Microbes. 2015;6(2):93-100.
Previous studies have shown Bismuth Subsalicylate (BSS) has antimicrobial properties, but few studies have addressed the mechanism of action. Furthermore, following BSS ingestion other bismuth salts form throughout the gastrointestinal tract including bismuth oxychloride (BiOCl) that also act upon enteric pathogens. To further understand the antimicrobial activity of bismuth in infectious diarrhea, the antimicrobial effect of BSS and BiOCl on Clostridium difficile, Salmonella, Shigella, Shiga toxin-producing Escherichia coli strains and norovirus (NoV) were measured. Bacterial enteric pathogens in pure culture or in human fecal material were exposed to 35mg/ml BSS or BiOCl with or without a vehicle suspension. BSS and BiOCl treated samples were quantified and visualized by transmission electron microscopy. To measure the effect on NoV, reduction of infectious murine NoV (MNV), a surrogate for human NoV, and Norwalk virus RNA levels were measured by viral plaque assay and RT-qPCR, respectively. BSS and BiOCl reduced bacterial growth by 3-9 logs in all strains with majority resulting in populations of <10 cfu/ml within 24 h. Similar results were found when fecal material was included. Microscopy images detected bismuth on bacterial membranes and within the bacterial organisms at 30 min post-treatment. At 8.8mg/ml BSS and BiOCl reduced infectivity of MNV significantly by 2.7 and 2.0 log after 24 h of exposure. In addition, both BSS and BiOCl slightly reduced the level of Norwalk replicon-bearing cells suggesting that bismuth may inhibit NoV in vivo. Collectively, our results confirm and build on existing data that BSS has antimicrobial properties against a wide-range of diarrhea-causing pathogens.


