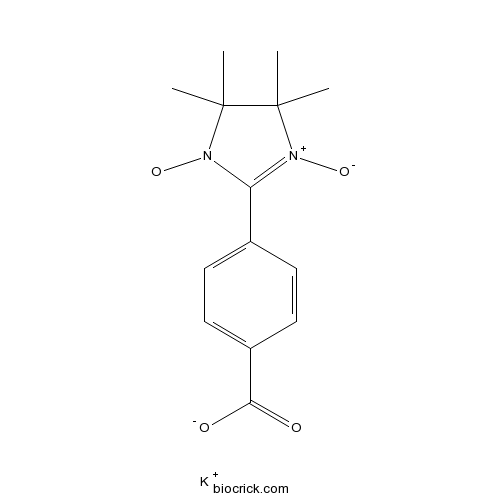
Carboxy-PTIO, potassium saltCAS# 148819-94-7 |
- GAP-134
Catalog No.:BCC1588
CAS No.:943134-39-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148819-94-7 | SDF | Download SDF |
| PubChem ID | 2733502 | Appearance | Powder |
| Formula | C14H16KN2O4 | M.Wt | 315.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in ethanol and to 10 mM in water | ||
| Chemical Name | 2-(4-Carboxyphenyl)-4,4,5,5-tetrame | ||
| SMILES | CC1(C([N+](=C(N1[O])C2=CC=C(C=C2)C(=O)[O-])[O-])(C)C)C.[K+] | ||
| Standard InChIKey | VYEUQMVIGXFZQU-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C14H18N2O4/c1-13(2)14(3,4)16(20)11(15(13)19)9-5-7-10(8-6-9)12(17)18/h5-8,19H,1-4H3,(H,17,18)/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A stable, water-soluble free radical that reacts stoichiometrically with NO. Almost twice as strong an inhibitor as N(ω)-nitroarginine or N-methylarginine. |

Carboxy-PTIO, potassium salt Dilution Calculator

Carboxy-PTIO, potassium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1708 mL | 15.8539 mL | 31.7078 mL | 63.4156 mL | 79.2695 mL |
| 5 mM | 0.6342 mL | 3.1708 mL | 6.3416 mL | 12.6831 mL | 15.8539 mL |
| 10 mM | 0.3171 mL | 1.5854 mL | 3.1708 mL | 6.3416 mL | 7.9269 mL |
| 50 mM | 0.0634 mL | 0.3171 mL | 0.6342 mL | 1.2683 mL | 1.5854 mL |
| 100 mM | 0.0317 mL | 0.1585 mL | 0.3171 mL | 0.6342 mL | 0.7927 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- Tyrphostin AG 879
Catalog No.:BCC4514
CAS No.:148741-30-4
- SB 204070
Catalog No.:BCC5752
CAS No.:148688-01-1
- L-733,060 hydrochloride
Catalog No.:BCC5707
CAS No.:148687-76-7
- GR 127935 hydrochloride
Catalog No.:BCC7081
CAS No.:148642-42-6
- Fmoc-Prolinol
Catalog No.:BCC2710
CAS No.:148625-77-8
- 3-O-Methylquercetin
Catalog No.:BCN1660
CAS No.:1486-70-0
- 3-O-Methylquercetin tetraacetate
Catalog No.:BCN1659
CAS No.:1486-69-7
- 3,5-Dihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1658
CAS No.:14858-07-2
- Pregabalin
Catalog No.:BCN2175
CAS No.:148553-50-8
- GRK2i
Catalog No.:BCC6048
CAS No.:148505-03-7
- MNS
Catalog No.:BCC3943
CAS No.:1485-00-3
- Bismuth Subsalicylate
Catalog No.:BCC3739
CAS No.:14882-18-9
- Rutamarin
Catalog No.:BCN7509
CAS No.:14882-94-1
- Ivabradine HCl
Catalog No.:BCC4350
CAS No.:148849-67-6
- YM 511
Catalog No.:BCC6002
CAS No.:148869-05-0
- HATU
Catalog No.:BCC2813
CAS No.:148893-10-1
- Fmoc-L-Arg(Aloc)2-OH
Catalog No.:BCC2564
CAS No.:148893-34-9
- 3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl-2-propenal
Catalog No.:BCC8600
CAS No.:148901-68-2
- Fmoc-Inp-OH
Catalog No.:BCC3266
CAS No.:148928-15-8
- Erythritol
Catalog No.:BCN1664
CAS No.:149-32-6
- Scopolamine butylbromide
Catalog No.:BCN5006
CAS No.:149-64-4
- Gallic acid
Catalog No.:BCN1668
CAS No.:149-91-7
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF.[Pubmed:8528578]
Br J Pharmacol. 1995 Sep;116(2):1906-10.
1. The effects of carboxy-PTIO, a scavenger of free radical nitric oxide (NO), were studied on endothelium-dependent relaxations of rat aorta and nitrergic nerve stimulation-induced relaxations of anococcygeus muscle and gastric fundus strips to test the hypothesis that endothelium-derived relaxing factor (EDRF) and the transmitter released by nitrergic nerves is free radical NO. 2. Carboxy-PTIO (10-300 microM) produced concentration-dependent reductions of relaxations elicited by exogenous NO, and relaxations mediated by EDRF released by acetylcholine and ATP in rings of rat aorta. The inhibitory effect of carboxy-PTIO was removed by washing the tissues. 3. In the rat anococcygeus muscle, carboxy-PTIO (10-300 microM) produced concentration-dependent reductions of relaxations to exogenous NO; however, in concentrations up to 2000 microM it did not reduce relaxations elicited by nitrergic nerve stimulation (1-2 Hz), in fact, concentrations of 300 microM or more slightly enhanced them. 4. In rat gastric fundus strips, carboxy-PTIO (100 and 300 microM) reduced relaxations to exogenous NO, but relaxations elicited by stimulation of the nitrergic component of non-adrenergic, non-cholinergic nerves were not affected. 5. These results suggest that EDRF is free radical NO and may be designated EDNO, but the transmitter released from nitrergic nerves does not appear to be identical to EDNO and may not be free radical NO.
Vasodilator effect of carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl in the coronary circulation: in vivo and in vitro studies.[Pubmed:7813579]
Eur J Pharmacol. 1994 Sep 1;262(1-2):55-63.
2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) derivatives, new radical forms of nitric oxide (NO) antagonists, are reported to react with NO and generate NO2 and 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl (PTI) derivatives. We found that carboxy-PTI, a water-soluble derivative of PTI, showed a potent vasodilator effect in the canine coronary artery system. In anesthetized dogs, intracoronary infusion of carboxy-PTI significantly increased the coronary flow in a dose-dependent manner without altering systemic hemodynamic variables. This coronary flow increasing effect of carboxy-PTI was not influenced by pretreatment with either NG-nitro-L-arginine methyl ester or 8-phenyltheophylline or autonomic blockade. However, the flow increasing effect of carboxy-PTI was abolished by reducing carboxy-PTI with ascorbic acid to a non-radical form of carboxy-PTI, indicating that carboxy-PTI shows its effect only in a radical form. In isolated canine coronary arterial rings, carboxy-PTI caused endothelium-independent relaxation. This relaxation response was significantly attenuated by pretreatment with methylene blue, an inhibitor of soluble guanylate cyclase. Thus, carboxy-PTI has an endothelium-independent coronary vasodilator effect in both large conduit arteries and small resistance vessels. The results of the in vitro experiment suggested that the activation of soluble guanylate cyclase of the vascular smooth muscle cell may be involved, at least in part, in the vasodilator mechanism of carboxy-PTI in large conduit arteries.
Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction.[Pubmed:8422387]
Biochemistry. 1993 Jan 26;32(3):827-32.
A labile inorganic free radical, nitric oxide (.NO), is produced by nitric oxide synthase from the substrate L-arginine in various cells and tissues. It acts as an endothelium-derived relaxing factor (EDRF) or as a neurotransmitter in vivo. We investigated the reactivity of stable radical compounds, imidazolineoxyl N-oxides such as 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO), carboxy-PTIO, and carboxymethoxy-PTIO against .NO/EDRF in both chemical and biological systems. By using electron spin resonance (ESR) spectroscopy, imidazolineoxyl N-oxides were found to react with .NO in a stoichiometric manner (PTIO/.NO = 1.0) in a neutral solution (sodium phosphate buffer, pH 7.4) with rate constants of approximately 10(4) M-1 s-1, resulting in the generation of NO2-/NO3- and imidazolineoxyls such as 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl (PTI), carboxy-PTI, or carboxymethoxy-PTI. Furthermore, the effects of imidazolineoxyl N-oxides on acetylcholine- or ATP-induced relaxation of the smooth muscle of rabbit aorta were tested. The vasorelaxations were inhibited by all three imidazolineoxyl N-oxides markedly. The inhibitory effects of carboxy-PTIO was almost 2-fold stronger than those of .NO synthesis inhibitors, N omega-nitro-L-arginine and N omega-monomethyl-L-arginine. Generation of EDRF/.NO was identified by reacting the PTIO in aortic strips and quantitating the reaction product with ESR spectroscopy. Thus, it was clarified that imidazolineoxyl N-oxide antagonize EDRF/.NO via a unique radical-radical reaction with .NO.


